Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2020 May 16;395(10236):1569-1578.
doi: 10.1016/S0140-6736(20)31022-9. Epub 2020 Apr 29.
Dingyu Zhang 2, Guanhua Du 3, Ronghui Du 4, Jianping Zhao 5, Yang Jin 6, Shouzhi Fu 7, Ling Gao 8, Zhenshun Cheng 9, Qiaofa Lu 10, Yi Hu 11, Guangwei Luo 12, Ke Wang 3, Yang Lu 3, Huadong Li 2, Shuzhen Wang 2, Shunan Ruan 2, Chengqing Yang 4, Chunlin Mei 4, Yi Wang 5, Dan Ding 5, Feng Wu 6, Xin Tang 6, Xianzhi Ye 7, Yingchun Ye 8, Bing Liu 9, Jie Yang 10, Wen Yin 11, Aili Wang 12, Guohui Fan 13, Fei Zhou 14, Zhibo Liu 14, Xiaoying Gu 13, Jiuyang Xu 15, Lianhan Shang 16, Yi Zhang 14, Lianjun Cao 17, Tingting Guo 17, Yan Wan 17, Hong Qin 18, Yushen Jiang 19, Thomas Jaki 20, Frederick G Hayden 21, Peter W Horby 22, Bin Cao 23, Chen Wang 24
Affiliations
- PMID: 32423584
- PMCID: PMC7190303
- DOI: 10.1016/S0140-6736(20)31022-9
Randomized Controlled Trial
Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial
Yeming Wang et al. Lancet. 2020.
Erratum in
- Department of Error.
[No authors listed] [No authors listed] Lancet. 2020 May 30;395(10238):1694. doi: 10.1016/S0140-6736(20)31204-6. Lancet. 2020. PMID: 32473675 Free PMC article. No abstract available.
Abstract
Background: No specific antiviral drug has been proven effective for treatment of patients with severe coronavirus disease 2019 (COVID-19). Remdesivir (GS-5734), a nucleoside analogue prodrug, has inhibitory effects on pathogenic animal and human coronaviruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro, and inhibits Middle East respiratory syndrome coronavirus, SARS-CoV-1, and SARS-CoV-2 replication in animal models.
Methods: We did a randomised, double-blind, placebo-controlled, multicentre trial at ten hospitals in Hubei, China. Eligible patients were adults (aged ≥18 years) admitted to hospital with laboratory-confirmed SARS-CoV-2 infection, with an interval from symptom onset to enrolment of 12 days or less, oxygen saturation of 94% or less on room air or a ratio of arterial oxygen partial pressure to fractional inspired oxygen of 300 mm Hg or less, and radiologically confirmed pneumonia. Patients were randomly assigned in a 2:1 ratio to intravenous remdesivir (200 mg on day 1 followed by 100 mg on days 2-10 in single daily infusions) or the same volume of placebo infusions for 10 days. Patients were permitted concomitant use of lopinavir-ritonavir, interferons, and corticosteroids. The primary endpoint was time to clinical improvement up to day 28, defined as the time (in days) from randomisation to the point of a decline of two levels on a six-point ordinal scale of clinical status (from 1=discharged to 6=death) or discharged alive from hospital, whichever came first. Primary analysis was done in the intention-to-treat (ITT) population and safety analysis was done in all patients who started their assigned treatment. This trial is registered with ClinicalTrials.gov, NCT04257656.
Findings: Between Feb 6, 2020, and March 12, 2020, 237 patients were enrolled and randomly assigned to a treatment group (158 to remdesivir and 79 to placebo); one patient in the placebo group who withdrew after randomisation was not included in the ITT population. Remdesivir use was not associated with a difference in time to clinical improvement (hazard ratio 1·23 [95% CI 0·87-1·75]). Although not statistically significant, patients receiving remdesivir had a numerically faster time to clinical improvement than those receiving placebo among patients with symptom duration of 10 days or less (hazard ratio 1·52 [0·95-2·43]). Adverse events were reported in 102 (66%) of 155 remdesivir recipients versus 50 (64%) of 78 placebo recipients. Remdesivir was stopped early because of adverse events in 18 (12%) patients versus four (5%) patients who stopped placebo early.
Interpretation: In this study of adult patients admitted to hospital for severe COVID-19, remdesivir was not associated with statistically significant clinical benefits. However, the numerical reduction in time to clinical improvement in those treated earlier requires confirmation in larger studies.
Funding: Chinese Academy of Medical Sciences Emergency Project of COVID-19, National Key Research and Development Program of China, the Beijing Science and Technology Project.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
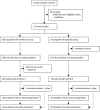
Figure 1
Trial profile
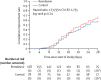
Figure 2
Time to clinical improvement in the intention-to-treat population Adjusted hazard ratio for randomisation stratification was 1·25 (95% CI 0·88–1·78). *Including deaths before day 28 as right censored at day 28, the number of patients without clinical improvement was still included in the number at risk.
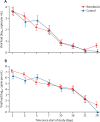
Figure 3
Viral load by quantitative PCR on the upper respiratory tract specimens (A) and lower respiratory tract specimens (B) Data are mean (SE). Results less than the lower limit of quantification of the PCR assay and greater than the limit of qualitative detection are imputed with half of actual value; results of patients with viral-negative RNA are imputed with 0 log10 copies per mL.
Comment in
- Remdesivir for COVID-19: challenges of underpowered studies.
Norrie JD. Norrie JD. Lancet. 2020 May 16;395(10236):1525-1527. doi: 10.1016/S0140-6736(20)31023-0. Epub 2020 Apr 29. Lancet. 2020. PMID: 32423580 Free PMC article. No abstract available. - COVID-19 Therapeutics: Making Sense of It All.
Bakare LS, Allen JM. Bakare LS, et al. AACN Adv Crit Care. 2020 Sep 15;31(3):239-249. doi: 10.4037/aacnacc2020792. AACN Adv Crit Care. 2020. PMID: 32668460 No abstract available. - Remdesivir and COVID-19: What are the implications for Africa?
Green RJ, Mustafa F, Sewlall N, Richards GA. Green RJ, et al. S Afr Med J. 2020 May 7;110(6):12942. S Afr Med J. 2020. PMID: 32880546 No abstract available. - Remdesivir and COVID-19.
Glaus MJ, Von Ruden S. Glaus MJ, et al. Lancet. 2020 Oct 3;396(10256):952. doi: 10.1016/S0140-6736(20)32021-3. Lancet. 2020. PMID: 33010831 Free PMC article. No abstract available. - Remdesivir and COVID-19.
Edwards JK, Cole SR, Adimora AA. Edwards JK, et al. Lancet. 2020 Oct 3;396(10256):953. doi: 10.1016/S0140-6736(20)32020-1. Lancet. 2020. PMID: 33010832 Free PMC article. No abstract available. - Remdesivir and COVID-19.
Wang LY, Cui JJ, Ouyang QY, Zhan Y, Guo CX, Yin JY. Wang LY, et al. Lancet. 2020 Oct 3;396(10256):953-954. doi: 10.1016/S0140-6736(20)32019-5. Lancet. 2020. PMID: 33010833 Free PMC article. No abstract available. - The place for remdesivir in COVID-19 treatment.
Young B, Tan TT, Leo YS. Young B, et al. Lancet Infect Dis. 2021 Jan;21(1):20-21. doi: 10.1016/S1473-3099(20)30911-7. Epub 2020 Nov 26. Lancet Infect Dis. 2021. PMID: 33248473 Free PMC article. No abstract available.
Similar articles
- Evaluation of the efficacy and safety of intravenous remdesivir in adult patients with severe COVID-19: study protocol for a phase 3 randomized, double-blind, placebo-controlled, multicentre trial.
Wang Y, Zhou F, Zhang D, Zhao J, Du R, Hu Y, Cheng Z, Gao L, Jin Y, Luo G, Fu S, Lu Q, Du G, Wang K, Lu Y, Fan G, Zhang Y, Liu Y, Ruan S, Liu W, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Wang Y, et al. Trials. 2020 May 24;21(1):422. doi: 10.1186/s13063-020-04352-9. Trials. 2020. PMID: 32448345 Free PMC article. - Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial.
Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, Ogbuagu O, Malhotra P, Mullane KM, Castagna A, Chai LYA, Roestenberg M, Tsang OTY, Bernasconi E, Le Turnier P, Chang SC, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wang H, Gaggar A, Brainard DM, McPhail MJ, Bhagani S, Ahn MY, Sanyal AJ, Huhn G, Marty FM; GS-US-540-5774 Investigators. Spinner CD, et al. JAMA. 2020 Sep 15;324(11):1048-1057. doi: 10.1001/jama.2020.16349. JAMA. 2020. PMID: 32821939 Free PMC article. Clinical Trial. - Remdesivir for the Treatment of Covid-19 - Final Report.
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members. Beigel JH, et al. N Engl J Med. 2020 Nov 5;383(19):1813-1826. doi: 10.1056/NEJMoa2007764. Epub 2020 Oct 8. N Engl J Med. 2020. PMID: 32445440 Free PMC article. Clinical Trial. - Compounds with Therapeutic Potential against Novel Respiratory 2019 Coronavirus.
Martinez MA. Martinez MA. Antimicrob Agents Chemother. 2020 Apr 21;64(5):e00399-20. doi: 10.1128/AAC.00399-20. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32152082 Free PMC article. Review. - Remdesivir in COVID-19: A critical review of pharmacology, pre-clinical and clinical studies.
Singh AK, Singh A, Singh R, Misra A. Singh AK, et al. Diabetes Metab Syndr. 2020 Jul-Aug;14(4):641-648. doi: 10.1016/j.dsx.2020.05.018. Epub 2020 May 12. Diabetes Metab Syndr. 2020. PMID: 32428865 Free PMC article. Review.
Cited by
- Identification of novel SARS-CoV-2 3CLpro inhibitors by molecular docking, in vitro assays, molecular dynamics simulations and DFT analyses.
Zong K, Wei C, Li W, Ruan J, Zhang S, Li J, Liu X, Zhao X, Cao R, Yan H, Li X. Zong K, et al. Front Pharmacol. 2024 Oct 30;15:1494953. doi: 10.3389/fphar.2024.1494953. eCollection 2024. Front Pharmacol. 2024. PMID: 39539614 Free PMC article. - Analysis of six consecutive waves of ICU-admitted COVID-19 patients: key findings and insights from a Portuguese population.
Von Rekowski CP, Pinto I, Fonseca TAH, Araújo R, Calado CRC, Bento L. Von Rekowski CP, et al. Geroscience. 2024 Nov 14. doi: 10.1007/s11357-024-01410-x. Online ahead of print. Geroscience. 2024. PMID: 39538084 - A systematic literature review on public health and healthcare resources for pandemic preparedness planning.
Beishuizen BHH, Stein ML, Buis JS, Tostmann A, Green C, Duggan J, Connolly MA, Rovers CP, Timen A. Beishuizen BHH, et al. BMC Public Health. 2024 Nov 11;24(1):3114. doi: 10.1186/s12889-024-20629-z. BMC Public Health. 2024. PMID: 39529010 Free PMC article. - Cannabinoid-Inspired Inhibitors of the SARS-CoV-2 Coronavirus 2'-_O_-Methyltransferase (2'-_O_-MTase) Non-Structural Protein (Nsp10-16).
Benjamin MM, Hanna GS, Dickinson CF, Choo YM, Wang X, Downs-Bowen JA, De R, McBrayer TR, Schinazi RF, Nielson SE, Hevel JM, Pandey P, Doerksen RJ, Townsend DM, Zhang J, Ye Z, Wyer S, Bialousow L, Hamann MT. Benjamin MM, et al. Molecules. 2024 Oct 28;29(21):5081. doi: 10.3390/molecules29215081. Molecules. 2024. PMID: 39519722 Free PMC article. - GPCR Inhibitors Have Antiviral Properties against JC Polyomavirus Infection.
Sandberg AL, Bond ACS, Bennett LJ, Craig SE, Winski DP, Kirkby LC, Kraemer AR, Kelly KG, Hess ST, Maginnis MS. Sandberg AL, et al. Viruses. 2024 Sep 30;16(10):1559. doi: 10.3390/v16101559. Viruses. 2024. PMID: 39459893 Free PMC article.
References
- Johns Hopkins University and Medicine COVID-19 map. Johns Hopkins Coronavirus Resource Centre. https://coronavirus.jhu.edu/map.html
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous