Management of cytotoxic chemotherapy-induced hand-foot syndrome - PubMed (original) (raw)
Management of cytotoxic chemotherapy-induced hand-foot syndrome
Johannes J M Kwakman et al. Oncol Rev. 2020.
Abstract
Improvements in systemic cancer treatments have resulted in more patients surviving for prolonged periods of time on treatment. This has made treatment-related toxicity and quality of life concerns increasingly relevant. Hand-foot syndrome (HFS) is a common skin reaction to systemic therapy that should be anticipated with chemotherapeutic treatments such as pegylated liposomal doxorubicin, docetaxel, and fluoropyrimidines. In this review we discuss current knowledge of the diagnosis, incidence, pathogenesis, and management of hand-foot syndrome (HFS). Although HFS is not life threatening, it can cause significant discomfort and impairment of function, especially in elderly patients, and may seriously impact quality of life. The incidence of HFS is dependent on the chemotherapeutic drug used, the treatment schedule, and the median duration of treatment. Effective measures for prevention and treatment of HFS include systemic and topical treatments, dose reductions, and switching to other drugs in the same class that are associated with lower rates of HFS. These approaches allow patients to continue cancer treatment while reducing negative impacts on quality of life. Awareness and early recognition are important to ensure timely treatment and avoidance of dose reductions or treatment discontinuation. We provide useful recommendations to guide the management of HFS in clinical practice.
Keywords: Hand-foot syndrome; docetaxel; doxorubicin; fluoropyrimidines; quality of life.
©Copyright: the Author(s).
Conflict of interest statement
Conflict of interests: Prof dr CJA Punt and prof dr M Koopman received research grants from the Dutch Colorectal Cancer Group and Servier outside the submitted work; dr JJM Kwakman received personal fees from Nordic Pharma for the conduct of this review, and research grants and lecture fees from Servier and Nordic Pharma outside the submitted work; dr YS Elshot received personal fees from Nordic Pharma for the conduct of this review. Nordic Pharma had no role in the design or writing of the review.
Figures
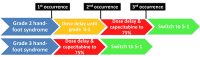
Figure 1.
Flowchart for treatment management of capecitabine-induced hand-foot syndrome.
Similar articles
- Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts.
von Moos R, Thuerlimann BJ, Aapro M, Rayson D, Harrold K, Sehouli J, Scotte F, Lorusso D, Dummer R, Lacouture ME, Lademann J, Hauschild A. von Moos R, et al. Eur J Cancer. 2008 Apr;44(6):781-90. doi: 10.1016/j.ejca.2008.01.028. Epub 2008 Mar 10. Eur J Cancer. 2008. PMID: 18331788 - Incidence and implications of chemotherapy related hand-foot syndrome.
Nikolaou V, Syrigos K, Saif MW. Nikolaou V, et al. Expert Opin Drug Saf. 2016 Dec;15(12):1625-1633. doi: 10.1080/14740338.2016.1238067. Epub 2016 Oct 8. Expert Opin Drug Saf. 2016. PMID: 27718746 Review. - Pyridoxine Is Effective for Preventing Hand-Foot Syndrome Induced by Pegylated Liposomal Doxorubicin for Multiple Myeloma: The Results of a Randomized Study.
Xiaozhe L, Meilan C, Beihui H, Junru L, Jingli G, Lifen K, Juan L. Xiaozhe L, et al. Integr Cancer Ther. 2022 Jan-Dec;21:15347354221140402. doi: 10.1177/15347354221140402. Integr Cancer Ther. 2022. PMID: 36510385 Free PMC article. Clinical Trial. - Analysis of drug-induced hand-foot syndrome using a spontaneous reporting system database.
Yoshida Y, Sasaoka S, Tanaka M, Matsumoto K, Inoue M, Satake R, Shimada K, Mukai R, Suzuki T, Iwata M, Goto F, Mori T, Mori K, Yoshimura T, Nakamura M. Yoshida Y, et al. Ther Adv Drug Saf. 2022 May 24;13:20420986221101963. doi: 10.1177/20420986221101963. eCollection 2022. Ther Adv Drug Saf. 2022. PMID: 35646307 Free PMC article. - Utility of cooling patches to prevent hand-foot syndrome caused by pegylated liposomal doxorubicin in breast cancer patients.
Zheng YF, Fu X, Wang XX, Sun XJ, He XD. Zheng YF, et al. World J Clin Cases. 2021 Nov 26;9(33):10075-10087. doi: 10.12998/wjcc.v9.i33.10075. World J Clin Cases. 2021. PMID: 34904077 Free PMC article.
Cited by
- Management of Skin Toxicities in Cancer Treatment: An Australian/New Zealand Perspective.
Ladwa R, Fogarty G, Chen P, Grewal G, McCormack C, Mar V, Kerob D, Khosrotehrani K. Ladwa R, et al. Cancers (Basel). 2024 Jul 12;16(14):2526. doi: 10.3390/cancers16142526. Cancers (Basel). 2024. PMID: 39061166 Free PMC article. Review. - Pharmaceutical and clinical studies of celecoxib topical hydrogel for management of chemotherapy-induced hand-foot syndrome.
Shayeganmehr D, Ramezannia F, Gharib B, Rezaeilaal A, Shahi F, Jafariazar Z, Afshar M. Shayeganmehr D, et al. Naunyn Schmiedebergs Arch Pharmacol. 2023 Jul;396(7):1571-1581. doi: 10.1007/s00210-022-02339-8. Epub 2022 Nov 24. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 36418469 Clinical Trial. - Evaluation of the impact of systemic dexamethasone dosage on docetaxel-induced hand-foot syndrome in patients with breast cancer.
Saito Y, Takekuma Y, Takahashi M, Oshino T, Sugawara M. Saito Y, et al. Sci Rep. 2024 Jun 18;14(1):14083. doi: 10.1038/s41598-024-64553-z. Sci Rep. 2024. PMID: 38890326 Free PMC article. - Adverse dermatoneurological events and impacts on daily activities of patients with gastrointestinal neoplasms undergoing chemotherapy.
Canille RMDS, Pinto MH, Galisteu KJ, Czorny RC, Bertolazzi LG, Faria TV. Canille RMDS, et al. Rev Bras Enferm. 2023 Jan 30;76(1):e20220161. doi: 10.1590/0034-7167-2022-0161. eCollection 2023. Rev Bras Enferm. 2023. PMID: 36722645 Free PMC article. - Systemic hypertension with VEGFR inhibitor (axitinib) therapy in cancer patients: A meta-analysis and systematic review of randomized controlled trials.
Dilli Babu A, Singh S, Thota A, Duhan S, Sainatham C, Gill H, Raghavakurup L, Tantry U, Bliden K, Gurbel P. Dilli Babu A, et al. Int J Cardiol Heart Vasc. 2024 May 5;52:101415. doi: 10.1016/j.ijcha.2024.101415. eCollection 2024 Jun. Int J Cardiol Heart Vasc. 2024. PMID: 38715853 Free PMC article.
References
- Saif MW, Elfiky AA. Identifying and treating fluoropyrimidine- associated hand-and-foot syndrome in white and nonwhite patients. J Support Oncol 2007;7:337-43. - PubMed
- CTCAE v5 November 27, 2017. Common Terminology Criteria for Adverse Events. National Cancer Institute. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.....
- Van Doorn L, Veelenturf S, Binkhorst L, et al. Capecitabine and the Risk of Fingerprint Loss. JAMA Oncol 2017;3:122-3. - PubMed
- Nagore E, Insa A, Sanmartin O. Antineoplastic therapyinduced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome: incidence, recognision and management. Am J Clin Dermatol 2000;1:225-34. - PubMed
- Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda). Eur J Oncol Nurs. 2004;8 Suppl 1:S31-40. - PubMed
LinkOut - more resources
Full Text Sources