Realising the therapeutic potential of neuroactive steroid modulators of the GABAA receptor - PubMed (original) (raw)
Realising the therapeutic potential of neuroactive steroid modulators of the GABAA receptor
Delia Belelli et al. Neurobiol Stress. 2019.
Abstract
In the 1980s particular endogenous metabolites of progesterone and of deoxycorticosterone were revealed to be potent, efficacious, positive allosteric modulators (PAMs) of the GABAA receptor (GABAAR). These reports were followed by the discovery that such steroids may be synthesised not only in peripheral endocrine glands, but locally in the central nervous system (CNS), to potentially act as paracrine, or autocrine "neurosteroid" messengers, thereby fine tuning neuronal inhibition. These discoveries triggered enthusiasm to elucidate the physiological role of such neurosteroids and explore whether their levels may be perturbed in particular psychiatric and neurological disorders. In preclinical studies the GABAAR-active steroids were shown to exhibit anxiolytic, anticonvulsant, analgesic and sedative properties and at relatively high doses to induce a state of general anaesthesia. Collectively, these findings encouraged efforts to investigate the therapeutic potential of neurosteroids and related synthetic analogues. However, following over 30 years of investigation, realising their possible medical potential has proved challenging. The recent FDA approval for the natural neurosteroid allopregnanolone (brexanolone) to treat postpartum depression (PPD) should trigger renewed enthusiasm for neurosteroid research. Here we focus on the influence of neuroactive steroids on GABA-ergic signalling and on the challenges faced in developing such steroids as anaesthetics, sedatives, analgesics, anticonvulsants, antidepressants and as treatments for neurodegenerative disorders.
Keywords: Allopregnanolone; GABAA receptor; Neurosteroid; Phasic inhibition; Tonic inhibition.
© 2019 Published by Elsevier Inc.
Figures
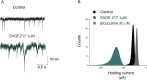
Fig. 1
SAGE-217 greatly enhances both phasic and tonic inhibition GABA A R in mouse thalamic VB neurons. A) GABAAR mIPSCs recorded at −70 mV from a representative VB neuron of a neonatal (P21) mouse horizontal slice before (Control, black trace) and following 10 min of SAGE 217 (1 μM, green trace). The mIPSCs appear as downward deflections from the baseline. Note the prolongation of the mIPSC decay by SAGE 217 and the simultaneous increase of the baseline noise. B) An all-points histogram of the holding current from the same recording depicted in A) under control conditions (black), following SAGE 217 (1 μM, green) and co-application of bicuculline (20 μM, grey). Note the large inward shift in the holding current after SAGE 217 (1 μM) and the reversal beyond the control holding current following bicuculline (20 μM), revealing a clear GABAAR tonic conductance. See Brown et al., 2015 for recording conditions. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
References
- Baldwin D.S., Aitchison K., Bateson A., Curran H.V., Davies S., Leonard B., Nutt D.J., Stephens D.N., Wilson S. Benzodiazepines: risks and benefits. A reconsideration. J. Psychopharmacol. 2013;27:967–971. - PubMed
- Barletta M., Alfaxalone: An old drug in a new formulation . 2019. Today's Veterinary Practice.https://todaysveterinarypractice.com/alfaxalone- an-old-drug-in- a-new-f... online.