Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial - PubMed (original) (raw)
Clinical Trial
. 2020 Jun 13;395(10240):1845-1854.
doi: 10.1016/S0140-6736(20)31208-3. Epub 2020 May 22.
Yu-Hua Li 2, Xu-Hua Guan 3, Li-Hua Hou 4, Wen-Juan Wang 5, Jing-Xin Li 5, Shi-Po Wu 4, Bu-Sen Wang 4, Zhao Wang 3, Lei Wang 3, Si-Yue Jia 5, Hu-Dachuan Jiang 5, Ling Wang 2, Tao Jiang 6, Yi Hu 6, Jin-Bo Gou 7, Sha-Bei Xu 8, Jun-Jie Xu 4, Xue-Wen Wang 9, Wei Wang 10, Wei Chen 11
Affiliations
- PMID: 32450106
- PMCID: PMC7255193
- DOI: 10.1016/S0140-6736(20)31208-3
Clinical Trial
Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial
Feng-Cai Zhu et al. Lancet. 2020.
Abstract
Background: A vaccine to protect against COVID-19 is urgently needed. We aimed to assess the safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 (Ad5) vectored COVID-19 vaccine expressing the spike glycoprotein of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) strain.
Methods: We did a dose-escalation, single-centre, open-label, non-randomised, phase 1 trial of an Ad5 vectored COVID-19 vaccine in Wuhan, China. Healthy adults aged between 18 and 60 years were sequentially enrolled and allocated to one of three dose groups (5 × 1010, 1 × 1011, and 1·5 × 1011 viral particles) to receive an intramuscular injection of vaccine. The primary outcome was adverse events in the 7 days post-vaccination. Safety was assessed over 28 days post-vaccination. Specific antibodies were measured with ELISA, and the neutralising antibody responses induced by vaccination were detected with SARS-CoV-2 virus neutralisation and pseudovirus neutralisation tests. T-cell responses were assessed by enzyme-linked immunospot and flow-cytometry assays. This study is registered with ClinicalTrials.gov, NCT04313127.
Findings: Between March 16 and March 27, 2020, we screened 195 individuals for eligibility. Of them, 108 participants (51% male, 49% female; mean age 36·3 years) were recruited and received the low dose (n=36), middle dose (n=36), or high dose (n=36) of the vaccine. All enrolled participants were included in the analysis. At least one adverse reaction within the first 7 days after the vaccination was reported in 30 (83%) participants in the low dose group, 30 (83%) participants in the middle dose group, and 27 (75%) participants in the high dose group. The most common injection site adverse reaction was pain, which was reported in 58 (54%) vaccine recipients, and the most commonly reported systematic adverse reactions were fever (50 [46%]), fatigue (47 [44%]), headache (42 [39%]), and muscle pain (18 [17%]. Most adverse reactions that were reported in all dose groups were mild or moderate in severity. No serious adverse event was noted within 28 days post-vaccination. ELISA antibodies and neutralising antibodies increased significantly at day 14, and peaked 28 days post-vaccination. Specific T-cell response peaked at day 14 post-vaccination.
Interpretation: The Ad5 vectored COVID-19 vaccine is tolerable and immunogenic at 28 days post-vaccination. Humoral responses against SARS-CoV-2 peaked at day 28 post-vaccination in healthy adults, and rapid specific T-cell responses were noted from day 14 post-vaccination. Our findings suggest that the Ad5 vectored COVID-19 vaccine warrants further investigation.
Funding: National Key R&D Program of China, National Science and Technology Major Project, and CanSino Biologics.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
Figure 1
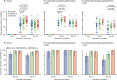
Specific T-cell response measured by ELISpot (A) The number of specific T cells with secretion of IFNγ at days 0, 14, and 28 in all participants, and stratified by pre-existing Ad5 neutralising antibody titres. (B) The proportion of positive ELISpot responders at days 14 and 28 post-vaccination in all participants, and stratified by pre-existing Ad5 neutralising antibody titres. IFN=interferon. PBMCs=peripheral blood mononuclear cells. Ad5=adenovirus type-5. ELISpot=enzyme-linked immunospot.
Figure 2
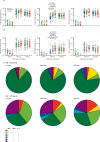
Flow cytometry with intracellular cytokine staining before and after vaccination (A) Percentage of cells secreting IFNγ, TNFα, and IL-2 from CD4+ T cells. (B) Percentage of cells secreting IFNγ, TNFα, and IL-2 from CD8+ T cells. (C) The proportion of CD4+ T cells and CD8+ T cells producing any combination of IFNγ, TNFα, and IL-2. The analyses are for 108 participants, with 36 in each dose group. IFN=interferon. TNF=tumour necrosis factor. IL=interleukin.
Comment in
- The starting line for COVID-19 vaccine development.
Lee N, McGeer A. Lee N, et al. Lancet. 2020 Jun 13;395(10240):1815-1816. doi: 10.1016/S0140-6736(20)31239-3. Epub 2020 May 28. Lancet. 2020. PMID: 32473680 Free PMC article. No abstract available. - Use of adenovirus type-5 vectored vaccines: a cautionary tale.
Buchbinder SP, McElrath MJ, Dieffenbach C, Corey L. Buchbinder SP, et al. Lancet. 2020 Oct 31;396(10260):e68-e69. doi: 10.1016/S0140-6736(20)32156-5. Epub 2020 Oct 19. Lancet. 2020. PMID: 33091364 Free PMC article. No abstract available.
Similar articles
- Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial.
Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, Hou LH, Li JX, Yang BF, Wang L, Wang WJ, Wu SP, Wang Z, Wu XH, Xu JJ, Zhang Z, Jia SY, Wang BS, Hu Y, Liu JJ, Zhang J, Qian XA, Li Q, Pan HX, Jiang HD, Deng P, Gou JB, Wang XW, Wang XH, Chen W. Zhu FC, et al. Lancet. 2020 Aug 15;396(10249):479-488. doi: 10.1016/S0140-6736(20)31605-6. Epub 2020 Jul 20. Lancet. 2020. PMID: 32702299 Free PMC article. Clinical Trial. - Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial.
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Folegatti PM, et al. Lancet. 2020 Aug 15;396(10249):467-478. doi: 10.1016/S0140-6736(20)31604-4. Epub 2020 Jul 20. Lancet. 2020. PMID: 32702298 Free PMC article. Clinical Trial. - Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial.
Wu S, Huang J, Zhang Z, Wu J, Zhang J, Hu H, Zhu T, Zhang J, Luo L, Fan P, Wang B, Chen C, Chen Y, Song X, Wang Y, Si W, Sun T, Wang X, Hou L, Chen W. Wu S, et al. Lancet Infect Dis. 2021 Dec;21(12):1654-1664. doi: 10.1016/S1473-3099(21)00396-0. Epub 2021 Jul 26. Lancet Infect Dis. 2021. PMID: 34324836 Free PMC article. Clinical Trial. - Adenoviral-vectored next-generation respiratory mucosal vaccines against COVID-19.
Afkhami S, Kang A, Jeyanathan V, Xing Z, Jeyanathan M. Afkhami S, et al. Curr Opin Virol. 2023 Aug;61:101334. doi: 10.1016/j.coviro.2023.101334. Epub 2023 May 11. Curr Opin Virol. 2023. PMID: 37276833 Free PMC article. Review. - The COVID-19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation.
Wang J, Peng Y, Xu H, Cui Z, Williams RO 3rd. Wang J, et al. AAPS PharmSciTech. 2020 Aug 5;21(6):225. doi: 10.1208/s12249-020-01744-7. AAPS PharmSciTech. 2020. PMID: 32761294 Free PMC article. Review.
Cited by
- Development of Bivalent mRNA Vaccines against SARS-CoV-2 Variants.
Li J, Liu Q, Liu J, Fang Z, Luo L, Li S, Lei Y, Li Z, Jin J, Xie R, Peng Y. Li J, et al. Vaccines (Basel). 2022 Oct 26;10(11):1807. doi: 10.3390/vaccines10111807. Vaccines (Basel). 2022. PMID: 36366316 Free PMC article. - Co-Created Messaging for Influenza Vaccination in a High-Risk Hispanic Community Provides Groundwork for COVID-19 Vaccine.
Long A, Mathew S, Alvarez KS, Smartt J, Shah M, Madden C, Perl TM, Cerise FP, Bhavan KP. Long A, et al. Health Equity. 2021 May 24;5(1):345-352. doi: 10.1089/heq.2020.0132. eCollection 2021. Health Equity. 2021. PMID: 34084986 Free PMC article. - SARS-CoV-2 Vaccination Improves Semen Quality in Men Recovered From COVID-19: A Retrospective Cohort Study.
Zhao Y, Wan Y, Hu X, Tong X, Xu B, Jiang X, Bai S, Cao C. Zhao Y, et al. Am J Mens Health. 2024 Jul-Aug;18(4):15579883241264120. doi: 10.1177/15579883241264120. Am J Mens Health. 2024. PMID: 39054777 Free PMC article. - Mucosal delivery of nanovaccine strategy against COVID-19 and its variants.
Lee J, Khang D. Lee J, et al. Acta Pharm Sin B. 2022 Nov 21;13(7):2897-925. doi: 10.1016/j.apsb.2022.11.022. Online ahead of print. Acta Pharm Sin B. 2022. PMID: 36438851 Free PMC article. Review. - Ongoing Clinical Trials of Vaccines to Fight against COVID-19 Pandemic.
Chakraborty C, Sharma AR, Bhattacharya M, Sharma G, Saha RP, Lee SS. Chakraborty C, et al. Immune Netw. 2021 Jan 19;21(1):e5. doi: 10.4110/in.2021.21.e5. eCollection 2021 Feb. Immune Netw. 2021. PMID: 33728098 Free PMC article. Review.
References
- WHO Coronavirus disease (COVID-2019) situation reports. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous