TLR2 and Dectin-1 Signaling in Mouse Hematopoietic Stem and Progenitor Cells Impacts the Ability of the Antigen Presenting Cells They Produce to Activate CD4 T Cells - PubMed (original) (raw)
TLR2 and Dectin-1 Signaling in Mouse Hematopoietic Stem and Progenitor Cells Impacts the Ability of the Antigen Presenting Cells They Produce to Activate CD4 T Cells
Alba Martínez et al. Cells. 2020.
Abstract
Microbial recognition by pattern recognition receptors (PRRs) expressed on hematopoietic stem and progenitor cells (HSPCs) not only activates myelopoiesis but also programs the function of the monocytes and macrophages they produce. For instance, changes in HSPC programming modify the ability of macrophages derived from them to produce inflammatory cytokines. While HSPCs exposed to a TLR2 agonist give rise to tolerized macrophages (lower proinflammatory cytokine production), HSPCs treated with Dectin-1 ligands produce trained macrophages (higher proinflammatory cytokine production). However, nothing is known about the impact of HSPC exposure to microbes on the function of antigen presenting cells (APCs). In this study we evaluated whether treatment of murine bone marrow HSPCs with a TLR2 or Dectin-1 ligand impacts the antigen presenting capacity of APCs derived from them in vitro. Following activation with microbial ligands or Candida albicans yeasts, APCs derived from TLR2/Dectin-1-programed HSPCs exhibit altered expression of MHCII (signal 1), co-stimulatory molecules (CD40, CD80 and CD86; signal 2) and cytokines (TNF-α, IL-6, IL-12 p40 and IL-2; signal 3). Moreover, APCs derived from TLR2/Dectin-1-programed HSPCs prime enhanced Th1 and Th17 responses, which are important for antifungal defense, in CD4 T cell cocultures. Overall, these results demonstrate for the first time that microbial detection by bone marrow HSPCs can modulate the adaptive immune response by inducing the production of APCs with an altered phenotype.
Keywords: CD4 T cells; Dectin-1; TLR2; antigen presenting cells; hematopoietic stem and progenitor cells; innate immune memory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure A1
(A) Percentage of fluorescently (TRITC) labeled zymosan particles present in the differentiation cultures shown in Figure 1 at different time points measured by flow cytometry. (B) CD11b and CD11c expression by the adherent cells recovered from the cultures at day 7 (C) Histograms and MFIs (numbers) corresponding to data shown in Figure 1B colormap. (D) Histograms and percentage of CD4+ T cells expressing CD44 or CD69 from cocultures obtained as indicated in Figure 2A. (E) Total CD4+ T cell numbers in the cocultures obtained as in Figure 2B, data represent means ± SD of triplicate cultures. Results shown are from one experiment representing three independent experiments.
Figure A2
(A) Histograms and MFIs (numbers) corresponding to data shown in Figure 3A colormaps. (B) Percentage of proliferating CD4+ T cells expressing CD69 from cocultures obtained as indicated in Figure 4A. (C) Total CD4+ T cell numbers in the cocultures obtained as in Figure 4B, data represent means ± SD of triplicate cultures. Results shown are from one experiment representing three independent experiments.
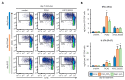
Figure 1
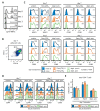
Pam3CSK4- or depleted zymosan-stimulated hematopoietic stem and progenitor cells (HSPCs) generate antigen presenting cells (APCs) with an altered phenotype in signals 1, 2, and 3. (A) Schematic protocol of in vitro HSPC differentiation. Purified Lin− cells from bone marrow of C57BL/6 mice were treated with Pam3CSK4, depleted zymosan, or nothing (none) during the first 24 h of culture, washed thoroughly to remove any remaining stimuli and then continued in culture with GM-CSF for a further 6 days to derive APCs. At day 6, adherent cells were recovered from the cultures, counted and plated at equal numbers for 24 h, and then stimulated with Pam3CSK4, depleted zymosan, or nothing (unstimulated) for 24 h to assess: (B) the expression of MHCII (signal 1) and costimulatory molecules (CD40, CD80, and CD86; signal 2) on CD11b+ CD11c+ APCs by flow cytometry (D0, day 0; N, none; P, Pam3CSK4; DZ, depleted zymosan), and (C) cytokine production (TNF-α, IL-6, IL-12 p40, and IL-2; signal 3) in the supernatants from the APC cultures by ELISA. Colormap is based on min-max values per row using the Mean Fluorescence Intensity (MFI) values obtained with the specific antibodies and the isotype controls (shown in Appendix A Figure A1C). Cytokine data represent means ± SD of triplicate cultures, * p < 0.05, ** p < 0.01, *** p < 0.001 with respect to day 0 none. Results shown are from one experiment representing three–five independent experiments.
Figure 2
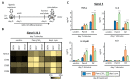
APCs derived from HSPCs stimulated with Pam3CSK4 or depleted zymosan prime enhanced Th1 and Th17 responses in OVA-specific CD4 T cell cocultures. APCs obtained as shown in Figure 1 were loaded with the OVA323–339 peptide and cocultured with CFSE-labeled CD4 T cells isolated from OT-II mice at 1:5 ratio (APC:T cell). Following three days of coculture, T cells were harvested for (A) flow cytometry analysis of proliferating CD4+ T cells and (B) cytokine production (IL-17A and IFN-γ) after 24 h of restimulation with PMA and ionomycin. Cytokine data was normalized by CD4 T cell numbers (shown in Figure A1E) and represent means ± SD of triplicate cultures, * p < 0.05, ** p < 0.01, *** p < 0.001 with respect to day 0 none. Results shown are from one experiment representing three independent experiments.
Figure 3
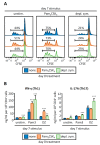
Pam3CSK4- or depleted zymosan-stimulated HSPCs generate APCs with an altered phenotype in Signals 1, 2 and 3 after C. albicans stimulation. Purified Lin− cells from bone marrow of C57BL/6 mice were treated with Pam3CSK4, depleted zymosan, or nothing (none) during the first 24 h of culture, washed thoroughly to remove any remaining stimuli, and then continued in culture with GM-CSF for a further six days to derive APCs. At day 6, adherent cells were recovered from the cultures, counted and plated at equal numbers for 24 h, and then stimulated with inactivated yeasts of C. albicans from the non-virulent strain PCA2 or the virulent strain ATCC 26555, or nothing (unstimulated) for 24 h to assess: (A) the expression of MHCII (signal 1) and costimulatory molecules (signal 2) on CD11b+ CD11c+ APCs by flow cytometry (D0, day 0; N, none; P, Pam3CSK4; DZ, depleted zymosan) and (B) the cytokine production (signal 3) in the supernatants from the APC cultures by ELISA. Colormap is based on min-max values per row using the MFI values obtained with the specific antibodies and the isotype controls (shown in Appendix A Figure A2A). Cytokine data represent means ± SD of triplicate cultures, * p < 0.05, ** p < 0.01, *** p < 0.001 with respect to day 0. Results shown are from one experiment representing three independent experiments.
Figure 4
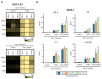
_C. albicans_-stimulated APCs derived from Pam3CSK4- or depleted zymosan-exposed HSPCs prime enhanced Th1 and Th17 responses in CD4 T cell cocultures. APCs obtained as in Figure 3 were cocultured with CFSE-labeled CD4 T cells isolated from naïve C57BL/6 mice at 1:5 ratio (APC:T cell). Following 4 days of coculture, T cells were harvested for (A) flow cytometry analysis of proliferating CD4+ T cells expressing CD44 and (B) cytokine production (IL-17A and IFN-γ) after 24 h of restimulation with PMA and ionomycin. Cytokine data were normalized by CD4 T cell numbers (shown in Figure A2C) and represent means ± SD of triplicate cultures, * p < 0.05, ** p < 0.01 with respect to day 0. Results shown are from one experiment representing three independent experiments.
Similar articles
- Dectin-1 Stimulation of Hematopoietic Stem and Progenitor Cells Occurs In Vivo and Promotes Differentiation Toward Trained Macrophages via an Indirect Cell-Autonomous Mechanism.
Bono C, Martínez A, Megías J, Gozalbo D, Yáñez A, Gil ML. Bono C, et al. mBio. 2020 Jun 23;11(3):e00781-20. doi: 10.1128/mBio.00781-20. mBio. 2020. PMID: 32576672 Free PMC article. - TLR2, TLR4 and Dectin-1 signalling in hematopoietic stem and progenitor cells determines the antifungal phenotype of the macrophages they produce.
Megías J, Martínez A, Yáñez A, Goodridge HS, Gozalbo D, Gil ML. Megías J, et al. Microbes Infect. 2016 May;18(5):354-63. doi: 10.1016/j.micinf.2016.01.005. Epub 2016 Jan 29. Microbes Infect. 2016. PMID: 26828664 - Systemic Candidiasis and TLR2 Agonist Exposure Impact the Antifungal Response of Hematopoietic Stem and Progenitor Cells.
Martínez A, Bono C, Megías J, Yáñez A, Gozalbo D, Gil ML. Martínez A, et al. Front Cell Infect Microbiol. 2018 Sep 3;8:309. doi: 10.3389/fcimb.2018.00309. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30234030 Free PMC article. - Role of Toll-like receptors in systemic Candida albicans infections.
Luisa Gil M, Murciano C, Yáñez A, Gozalbo D. Luisa Gil M, et al. Front Biosci (Landmark Ed). 2016 Jan 1;21(2):278-302. doi: 10.2741/4388. Front Biosci (Landmark Ed). 2016. PMID: 26709773 Review. - Microbial Sensing by Hematopoietic Stem and Progenitor Cells.
Barman PK, Goodridge HS. Barman PK, et al. Stem Cells. 2022 Mar 3;40(1):14-21. doi: 10.1093/stmcls/sxab007. Stem Cells. 2022. PMID: 35511863 Free PMC article. Review.
Cited by
- The Interplay between the Gut Microbiome and the Immune System in the Context of Infectious Diseases throughout Life and the Role of Nutrition in Optimizing Treatment Strategies.
Wiertsema SP, van Bergenhenegouwen J, Garssen J, Knippels LMJ. Wiertsema SP, et al. Nutrients. 2021 Mar 9;13(3):886. doi: 10.3390/nu13030886. Nutrients. 2021. PMID: 33803407 Free PMC article. Review. - CD3ε of a pan T cell marker involved in mouse Aspergillus fumigatus keratitis.
Shi WH, Wang LM, Yan HJ, Liu SL, Yang X, Yang XJ, Che CY. Shi WH, et al. Int J Ophthalmol. 2024 Apr 18;17(4):616-624. doi: 10.18240/ijo.2024.04.03. eCollection 2024. Int J Ophthalmol. 2024. PMID: 38638265 Free PMC article. - From structure to function - Ligand recognition by myeloid C-type lectin receptors.
Fischer S, Stegmann F, Gnanapragassam VS, Lepenies B. Fischer S, et al. Comput Struct Biotechnol J. 2022 Oct 20;20:5790-5812. doi: 10.1016/j.csbj.2022.10.019. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36382179 Free PMC article. Review. - Dectin-1 Signaling Update: New Perspectives for Trained Immunity.
Mata-Martínez P, Bergón-Gutiérrez M, Del Fresno C. Mata-Martínez P, et al. Front Immunol. 2022 Feb 14;13:812148. doi: 10.3389/fimmu.2022.812148. eCollection 2022. Front Immunol. 2022. PMID: 35237264 Free PMC article. Review. - Single-Cell RNA Sequencing of Tocilizumab-Treated Peripheral Blood Mononuclear Cells as an in vitro Model of Inflammation.
Zarinsefat A, Hartoularos G, Rychkov D, Rashmi P, Chandran S, Vincenti F, Yee CJ, Sarwal MM. Zarinsefat A, et al. Front Genet. 2021 Jan 5;11:610682. doi: 10.3389/fgene.2020.610682. eCollection 2020. Front Genet. 2021. PMID: 33469465 Free PMC article.
References
- Yáñez A., Hassanzadeh-Kiabi N., Ng M.Y., Megías J., Subramanian A., Liu G.Y., Underhill D.M., Gil M.L., Goodridge H.S. Detection of a TLR2 agonist by hematopoietic stem and progenitor cells impacts the function of the macrophages they produce. Eur. J. Immunol. 2013;43:2114–2125. doi: 10.1002/eji.201343403. - DOI - PMC - PubMed
- Megías J., Martínez A., Yáñez A., Goodridge H.S., Gozalbo D., Gil M.L. TLR2, TLR4 and Dectin-1 signalling in hematopoietic stem and progenitor cells determines the antifungal phenotype of the macrophages they produce. Microbes Infect. 2016;18:354–363. doi: 10.1016/j.micinf.2016.01.005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous