Discovery of the FDA-approved drugs bexarotene, cetilistat, diiodohydroxyquinoline, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system - PubMed (original) (raw)
doi: 10.1016/j.phrs.2020.104960. Epub 2020 May 28.
Jasper F W Chan 2, Kenn K H Chik 1, Chris C Y Chan 1, Jessica O L Tsang 1, Ronghui Liang 1, Jianli Cao 1, Kaiming Tang 1, Lin-Lei Chen 1, Kun Wen 3, Jian-Piao Cai 1, Zi-Wei Ye 1, Gang Lu 4, Hin Chu 1, Dong-Yan Jin 5, Kwok-Yung Yuen 6
Affiliations
- PMID: 32473310
- PMCID: PMC7254006
- DOI: 10.1016/j.phrs.2020.104960
Discovery of the FDA-approved drugs bexarotene, cetilistat, diiodohydroxyquinoline, and abiraterone as potential COVID-19 treatments with a robust two-tier screening system
Shuofeng Yuan et al. Pharmacol Res. 2020 Sep.
Abstract
Coronavirus Disease 2019 (COVID-19) caused by the emerging severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is associated with a crude case fatality rate of about 0.5-10 % depending on locality. A few clinically approved drugs, such as remdesivir, chloroquine, hydroxychloroquine, nafamostat, camostat, and ivermectin, exhibited anti-SARS-CoV-2 activity in vitro and/or in a small number of patients. However, their clinical use may be limited by anti-SARS-CoV-2 50 % maximal effective concentrations (EC50) that exceeded their achievable peak serum concentrations (Cmax), side effects, and/or availability. To find more immediately available COVID-19 antivirals, we established a two-tier drug screening system that combines SARS-CoV-2 enzyme-linked immunosorbent assay and cell viability assay, and applied it to screen a library consisting 1528 FDA-approved drugs. Cetilistat (anti-pancreatic lipase), diiodohydroxyquinoline (anti-parasitic), abiraterone acetate (synthetic androstane steroid), and bexarotene (antineoplastic retinoid) exhibited potent in vitro anti-SARS-CoV-2 activity (EC50 1.13-2.01 μM). Bexarotene demonstrated the highest Cmax:EC50 ratio (1.69) which was higher than those of chloroquine, hydroxychloroquine, and ivermectin. These results demonstrated the efficacy of the two-tier screening system and identified potential COVID-19 treatments which can achieve effective levels if given by inhalation or systemically depending on their pharmacokinetics.
Keywords: Abiraterone; Abiraterone acetate (PubChem CID: 9821849); Antiviral; Bexarotene; Bexarotene (PubChem CID: 82146); COVID-19; Cetilistat; Cetilistat (PubChem CID: 9952916); Coronavirus; Diiodohydroxyquinoline; Diiodohydroxyquinoline (PubChem CID: 3728); Library; SARS-CoV-2; Treatment.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
JFWC has received travel grants from Pfizer Corporation Hong Kong and Astellas Pharma Hong Kong Corporation Limited, and was an invited speaker for Gilead Sciences Hong Kong Limited and Luminex Corporation. The other authors declared no conflict of interests. The funding sources had no role in study design, data collection, analysis or interpretation or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Figures
Graphical abstract
Fig. 1
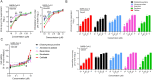
Development of a two-tier system for anti-SARS-CoV-2 drug compound screening. VeroE6 cells seeded in a 96-well plate were infected with SARS-CoV-2 of various multiplicities of infection (MOIs)) as indicated, followed by phosphate buffered saline (PBS) wash and replacement of fresh Dulbecco’s modified eagle medium (DMEM). Various time points of data collection were performed for (A) Cytopathic effects (CPE) observed by a bright-field at 20× magnification. (B) Cell viability of each treatment group normalized with that of the mock-infected cells. (C) Cell culture supernatant was collected at the indicated time points with viral copy number determined by quantitative RT-PCR (qRT-PCR). (D) The supernatant was concomitantly applied for ELISA to measure the SARS-CoV-2-nucleoprotein (NP) protein amount. The experiments were carried out in triplicate. The results are shown as mean ± standard deviation.
Fig. 2
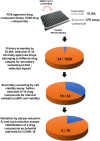
Schematic representation of the study design. Using the newly established two-tier drug compound screening system, an FDA-approved drug compound library consisting 1528 drug compounds was screened for potential anti-SARS-CoV-2 agents. Primary screening by ELISA identified 34 drug compounds with 4-fold reduction in the detection signal. Secondary screening by cell viability assay further selected 19 of the 34 drug compounds that exhibited ≥90 % cell viability. Four drug compounds were then prioritized for cytotoxicity and antiviral activity evaluation by plaque reduction and viral load reduction assays.
Fig. 3
Identification of four FDA-approved drug compounds with potent anti-SARS-CoV-2 activity. Chemical structures of the selected compounds and photos of plaque reduction assay are shown: (A) cetilistat, (B) diiodohydroxyquinoline, (C) abiraterone acetate, and (D) bexarotene.
Fig. 4
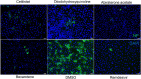
Immunofluorescence staining showing the anti-SARS-CoV-2 effects of the four selected drug compounds. Fixation and staining were performed on SARS-CoV-2-infected (MOI = 0.100) VeroE6 cells after treatment with cetilistat, diiodohydroxyquinoline, abiraterone acetate, or bexarotene (10μM each) and incubated at 37 °C with 5% CO2 for 24 h. The SARS-CoV-2-N antigens and cell nuclei (DAPI) were stained in green and blue, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Fig. 5
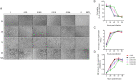
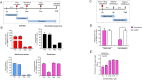
Characterization of the anti-SARS-CoV-2 activity of each of the four selected drug compounds in vitro. (A) VeroE6 and (B) Caco2 cells were infected with SARS-CoV-2 and treated with different concentrations of the selected drug compounds as indicated. The viral load under each condition was collected at 48 hpi for viral load reduction assay by qRT-PCR. Intracellular viral loads were normalized by human β-actin. (C) VeroE6 cells were infected with SARS-CoV-2 and treated with different concentrations of the selected drug compounds as indicated and evaluated by the CPE inhibition assay at 72 hpi. Remdesivir was used as a positive control in all of these experiments. One-way ANOVA was used to compare the treatment groups with the 0μM (negative control) group. *P indicates < 0.05 and ** indicates P < 0.01 (Student’s t-test). All the experiments were performed in triplicate and replicated twice. The results are shown as mean ± standard deviations.
Fig. 6
Anti-SARS-CoV-2 modes of action of the four selected drug compounds. (A) Time-of-drug-addition assay was performed to determine the steps of the viral replication cycle targeted by each of the four identified drug compounds. The assay was conducted at 37 °C. (B) Viral load in the treated VeroE6 cell culture supernatants normalized by DMSO as control. (C) Virus entry assay with or without bexarotene addition. Viral attachment was performed at 4℃ and then shifted to 37℃ to enable virus internalization. (D) Intracellular viral load in the treated VeroE6 cells normalized by DMSO as control. (E) Viral load reduction assay showing the dose-dependent anti-MERS-CoV activity of bexarotene (10μM). VeroE6 cells were infected with MERS-CoV (MOI = 0.01) and treated with bexarotene, and the culture supernatant was then collected at 48 h post-inoculation for viral load quantitation by qRT-PCR. *P indicates < 0.05 and ** indicates P < 0.01 (Student’s t-test). All the experiments were performed in triplicate and replicated twice. The results are shown as mean ± standard deviations.
Similar articles
- The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro.
Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. Caly L, et al. Antiviral Res. 2020 Jun;178:104787. doi: 10.1016/j.antiviral.2020.104787. Epub 2020 Apr 3. Antiviral Res. 2020. PMID: 32251768 Free PMC article. - Broad-Spectrum Host-Based Antivirals Targeting the Interferon and Lipogenesis Pathways as Potential Treatment Options for the Pandemic Coronavirus Disease 2019 (COVID-19).
Yuan S, Chan CC, Chik KK, Tsang JO, Liang R, Cao J, Tang K, Cai JP, Ye ZW, Yin F, To KK, Chu H, Jin DY, Hung IF, Yuen KY, Chan JF. Yuan S, et al. Viruses. 2020 Jun 10;12(6):628. doi: 10.3390/v12060628. Viruses. 2020. PMID: 32532085 Free PMC article. - In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication.
Touret F, Gilles M, Barral K, Nougairède A, van Helden J, Decroly E, de Lamballerie X, Coutard B. Touret F, et al. Sci Rep. 2020 Aug 4;10(1):13093. doi: 10.1038/s41598-020-70143-6. Sci Rep. 2020. PMID: 32753646 Free PMC article. - Prioritization of Anti-SARS-Cov-2 Drug Repurposing Opportunities Based on Plasma and Target Site Concentrations Derived from their Established Human Pharmacokinetics.
Arshad U, Pertinez H, Box H, Tatham L, Rajoli RKR, Curley P, Neary M, Sharp J, Liptrott NJ, Valentijn A, David C, Rannard SP, O'Neill PM, Aljayyoussi G, Pennington SH, Ward SA, Hill A, Back DJ, Khoo SH, Bray PG, Biagini GA, Owen A. Arshad U, et al. Clin Pharmacol Ther. 2020 Oct;108(4):775-790. doi: 10.1002/cpt.1909. Epub 2020 Jun 14. Clin Pharmacol Ther. 2020. PMID: 32438446 Free PMC article. Review. - Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: A narrative review.
Hashem AM, Alghamdi BS, Algaissi AA, Alshehri FS, Bukhari A, Alfaleh MA, Memish ZA. Hashem AM, et al. Travel Med Infect Dis. 2020 May-Jun;35:101735. doi: 10.1016/j.tmaid.2020.101735. Epub 2020 May 6. Travel Med Infect Dis. 2020. PMID: 32387694 Free PMC article. Review.
Cited by
- COVID-19 drug discovery and treatment options.
Chan JF, Yuan S, Chu H, Sridhar S, Yuen KY. Chan JF, et al. Nat Rev Microbiol. 2024 Jul;22(7):391-407. doi: 10.1038/s41579-024-01036-y. Epub 2024 Apr 15. Nat Rev Microbiol. 2024. PMID: 38622352 Review. - Coordination chemistry suggests that independently observed benefits of metformin and Zn2+ against COVID-19 are not independent.
Lockwood TD. Lockwood TD. Biometals. 2024 Aug;37(4):983-1022. doi: 10.1007/s10534-024-00590-5. Epub 2024 Apr 5. Biometals. 2024. PMID: 38578560 Free PMC article. - Potential Use of ChatGPT in Responding to Patient Questions and Creating Patient Resources.
Reynolds K, Tejasvi T. Reynolds K, et al. JMIR Dermatol. 2024 Mar 6;7:e48451. doi: 10.2196/48451. JMIR Dermatol. 2024. PMID: 38446541 Free PMC article. - Lessons Learned From Limited Overlap of 15 In Vitro COVID-19 Drug Repurposing Screens.
Tomezsko PJ, Phillipson CW, Walsh ME. Tomezsko PJ, et al. Health Secur. 2023 Jul-Aug;21(4):249-257. doi: 10.1089/hs.2022.0132. Epub 2023 May 17. Health Secur. 2023. PMID: 37196212 Free PMC article. - Outpatient medications associated with protection from COVID-19 hospitalization.
Sandhu HS, Lambert J, Steckler Z, Park L, Stromberg A, Ramirez J, Yang CJ. Sandhu HS, et al. PLoS One. 2023 Mar 31;18(3):e0282961. doi: 10.1371/journal.pone.0282961. eCollection 2023. PLoS One. 2023. PMID: 37000808 Free PMC article.
References
- World Health Organization . 2020. Coronavirus Disease 2019 (COVID-19) Situation Report – 77.https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous