Protecting HaCaT cells from ionizing radiation using persimmon tannin- Aloe gel composite - PubMed (original) (raw)
Protecting HaCaT cells from ionizing radiation using persimmon tannin- Aloe gel composite
Xi Qian et al. Pharm Biol. 2020 Dec.
Abstract
Context: Persimmon tannin (extract of Diospyros kaki L.f [Ebenaceae]) and Aloe gel **(**extract of Aloe vera (L.) Burm.f. [Asphodelaceae]) are known as anti-radiation agents. However, radiation resistance of the persimmon tannin-Aloe gel composite remains inconclusive.Objective: To investigate the capacity of the persimmon tannin-Aloe gel composite to protect against ionising radiation at the cellular level.Materials and methods: HaCaT (human epidermal keratinocytes) cells were pre-treated with PT-A-1 (the mass ratio of persimmon tannin and Aloe gel was 2:1) or the single component (persimmon tannin or Aloe gel) at various concentrations (0, 50, 100, 200, 400, 800 μg/mL. Control group: medium with no HaCaT cells), and then radiated with X-rays (radiation dose: 4, 8, 12, 16, and 20 Gy). Cell viability, cell apoptosis, and radiation-induced intracellular reactive oxygen species (ROS) generation were analysed by CCK-8, Hoechst 33258 staining/flow cytometry, and 2',7'-dichlorfluorescein diacetate (DCFH-DA) assay, respectively, for 12 or 24 h incubation after radiation.Results: The optimal radiation dose and post-radiation incubation period were determined to be 8 Gy and 12 h. CCK-8 activity detection showed that the cell activity was 77.85% (p < 0.05, IC50 = 55.67 μg/mL). The apoptotic rate was the lowest (4.32%) at 200 μg/mL of PT-A-1 towards HaCaT cells. ROS production was the most effectively suppressed by 200 μg/mL PT-A-1 towards HaCaT cells.Discussion and conclusions: The persimmon tannin-Aloe gel composite has good radioprotective effect, and which will facilitate its clinic application as a potential natural anti-radiation agent in future.
Keywords: Human epidermal keratinocytes cells; Radiation resistance; X-rays.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Figure 1.
The HaCaT cells morphology with different ingredients at a concentration of 200 μg/mL after 8 Gy radiation pre-treatment for 12 h incubation (400×).
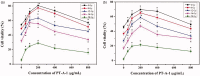
Figure 2.
Cellular viability (%) of HaCaT cells with PT-A-1 for different concentrations and different radiation doses for 12 h incubation after X-ray irradiation (a); Cellular viability (%) of HaCaT cells with PT-A-1 for different concentrations and different radiation doses for 24 h incubation after X-ray irradiation (b). All above values are presented as the median from analysis of three independent experiments and the error bars indicate standard deviation (n = 3, p < 0.05). Symbol* represents p < 0.05 vs. blank group (Concentration of 0 g/mL).
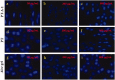
Figure 3.
Hoechst 33258 fluorescent dyeing of HaCat cells pre-treated with different concentrations of PT-A, PT, and Aloe gel materials for 12 h incubation after 8 Gy X-ray irradiation (200×).
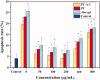
Figure 4.
Flow cytometry detecting the apoptosis rate of HaCaT cells pre-treated with different concentrations of PT-A, PT, and Aloe gel materials for 12 h incubation after 8 Gy X-ray irradiation. All above values are presented as the median from analysis of three independent experiments and the error bars indicate standard deviation (n = 3), Symbol* represents p < 0.05 vs. Control group.
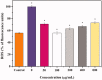
Figure 5.
The inhibition effects of PT-A-1 pre-treatment prior to radiation with 8 Gy X-ray irradiation on the production of intracellular reactive oxygen species in HaCat cells. All above values are presented as the median from analysis of three independent experiments and the error bars indicate standard deviation (n = 3), Symbol* represents p < 0.05 vs. Control group.
Similar articles
- Effects of Persimmon Tannin-Aloe vera Composite on Cytotoxic Activities, and Radioprotection Against X-rays Irradiated in Human Hepatoma and Hepatic Cells.
Liu ZG, Qian X, Wang ZM, Ning JL, Qin CK, Huang ZM, Li YM, He N, Lin DH, Zhou ZD, Li GY. Liu ZG, et al. J Biomed Nanotechnol. 2021 Oct 1;17(10):2043-2052. doi: 10.1166/jbn.2021.3177. J Biomed Nanotechnol. 2021. PMID: 34706804 - Extraction, purification and anti-radiation activity of persimmon tannin from Diospyros kaki L.f.
Zhou Z, Huang Y, Liang J, Ou M, Chen J, Li G. Zhou Z, et al. J Environ Radioact. 2016 Oct;162-163:182-188. doi: 10.1016/j.jenvrad.2016.05.034. Epub 2016 Jun 3. J Environ Radioact. 2016. PMID: 27267156 - Inactivation of pathogenic viruses by plant-derived tannins: strong effects of extracts from persimmon (Diospyros kaki) on a broad range of viruses.
Ueda K, Kawabata R, Irie T, Nakai Y, Tohya Y, Sakaguchi T. Ueda K, et al. PLoS One. 2013;8(1):e55343. doi: 10.1371/journal.pone.0055343. Epub 2013 Jan 25. PLoS One. 2013. PMID: 23372851 Free PMC article. - Activity and potential mechanisms of action of persimmon tannins according to their structures: A review.
Wang R, Shi X, Li K, Bunker A, Li C. Wang R, et al. Int J Biol Macromol. 2023 Jul 1;242(Pt 3):125120. doi: 10.1016/j.ijbiomac.2023.125120. Epub 2023 May 30. Int J Biol Macromol. 2023. PMID: 37263329 Review. - Persimmon (Diospyros kaki L.) leaves: a review on traditional uses, phytochemistry and pharmacological properties.
Xie C, Xie Z, Xu X, Yang D. Xie C, et al. J Ethnopharmacol. 2015 Apr 2;163:229-40. doi: 10.1016/j.jep.2015.01.007. Epub 2015 Jan 28. J Ethnopharmacol. 2015. PMID: 25637828 Review.
Cited by
- Topically applied fullerenols protect against radiation dermatitis by scavenging reactive oxygen species.
Yin H, Gao Y, Chen W, Tang C, Zhu Z, Li K, Xia S, Han C, Ding X, Ruan F, Tian H, Zhu C, Xie S, Zuo Z, Liao L, He C. Yin H, et al. Discov Nano. 2023 Aug 15;18(1):101. doi: 10.1186/s11671-023-03869-7. Discov Nano. 2023. PMID: 37581715 Free PMC article. - Potential Utilisation of Theobroma cacao Pod Husk Extract: Protective Capability Evaluation Against Pollution Models and Formulation into Niosomes.
Chriscensia E, Nathanael J, Perwitasari U, Putra ABN, Adiyanto SA, Hartrianti P. Chriscensia E, et al. Trop Life Sci Res. 2024 Jul;35(2):107-140. doi: 10.21315/tlsr2024.35.2.6. Epub 2024 Jul 31. Trop Life Sci Res. 2024. PMID: 39234471 Free PMC article. - Computational and Experimental Investigation of the Selective Adsorption of Indium/Iron Ions by the Epigallocatechin Gallate Monomer.
Liu Z, Wang Z, Gan W, Liu S, Zhang J, Ran Z, Wu C, Hu C, Wang D, Chen T, Li G. Liu Z, et al. Materials (Basel). 2022 Nov 21;15(22):8251. doi: 10.3390/ma15228251. Materials (Basel). 2022. PMID: 36431735 Free PMC article. - Activation of NRF2 by topical apocarotenoid treatment mitigates radiation-induced dermatitis.
Schmidlin CJ, Rojo de la Vega M, Perer J, Zhang DD, Wondrak GT. Schmidlin CJ, et al. Redox Biol. 2020 Oct;37:101714. doi: 10.1016/j.redox.2020.101714. Epub 2020 Sep 4. Redox Biol. 2020. PMID: 32927319 Free PMC article. - Towards unravelling biological mechanisms behind radiation-induced oral mucositis via mass spectrometry-based proteomics.
Subedi P, Huber K, Sterr C, Dietz A, Strasser L, Kaestle F, Hauck SM, Duchrow L, Aldrian C, Monroy Ordonez EB, Luka B, Thomsen AR, Henke M, Gomolka M, Rößler U, Azimzadeh O, Moertl S, Hornhardt S. Subedi P, et al. Front Oncol. 2023 Jun 13;13:1180642. doi: 10.3389/fonc.2023.1180642. eCollection 2023. Front Oncol. 2023. PMID: 37384298 Free PMC article.
References
- Abernathy LM, Fountain MD, Rothstein SE, David JM, Yunker CK, Rakowski J, Lonardo F, Joiner MC, Hillman GG.. 2015. Soy isoflavones promote radioprotection of normal lung tissue by inhibition of radiation-induced activation of macrophages and neutrophils. J Thorac Oncol. 10(12):1703–1712. - PMC - PubMed
- Bala S, Chugh NA, Bansal SC, Garg ML, Koul A.. 2018. Radiomodulatory effects of Aloe vera on hepatic and renal tissues of X-ray irradiated mice. Mutat Res. 811:1–15. - PubMed
- Burmeister WP. 2000. Structural changes in a cryo-cooled protein crystal owing to radiation damage. Acta Crystallogr D Biol Crystallogr. 56(Pt 3):328–341. - PubMed
- Chavan Y, Singhal RS.. 2013. Ultrasound-assisted extraction (UAE) of bioactives from arecanut (Areca catechu L.) and optimization study using response surface methodology. Innov Food SCI Emerg. 17:106–113.
MeSH terms
Substances
Grants and funding
This work was supported by National Natural Science Foundation of China [Nos. 81760534 and 51961010], Guangxi Key Research and Development Program [Nos. Guike 2018AB38016 and Guike AB16380278], the Natural Science Foundations of Guangxi Province [No. 2016GXNSFGA380001].
LinkOut - more resources
Full Text Sources