Essential Role of Hemoglobin βCys93 in Cardiovascular Physiology - PubMed (original) (raw)
Review
Essential Role of Hemoglobin βCys93 in Cardiovascular Physiology
Richard T Premont et al. Physiology (Bethesda). 2020.
Abstract
The supply of oxygen to tissues is controlled by microcirculatory blood flow. One of the more surprising discoveries in cardiovascular physiology is the critical dependence of microcirculatory blood flow on a single conserved cysteine within the β-subunit (βCys93) of hemoglobin (Hb). βCys93 is the primary site of Hb _S_-nitrosylation [i.e., _S_-nitrosothiol (SNO) formation to produce _S_-nitrosohemoglobin (SNO-Hb)]. Notably, _S_-nitrosylation of βCys93 by NO is favored in the oxygenated conformation of Hb, and deoxygenated Hb releases SNO from βCys93. Since SNOs are vasodilatory, this mechanism provides a physiological basis for how tissue hypoxia increases microcirculatory blood flow (hypoxic autoregulation of blood flow). Mice expressing βCys93A mutant Hb (C93A) have been applied to understand the role of βCys93, and RBCs more generally, in cardiovascular physiology. Notably, C93A mice are unable to effect hypoxic autoregulation of blood flow and exhibit widespread tissue hypoxia. Moreover, reactive hyperemia (augmentation of blood flow following transient ischemia) is markedly impaired. C93A mice display multiple compensations to preserve RBC vasodilation and overcome tissue hypoxia, including shifting SNOs to other thiols on adult and fetal Hbs and elsewhere in RBCs, and growing new blood vessels. However, compensatory vasodilation in C93A mice is uncoupled from hypoxic control, both peripherally (e.g., predisposing to ischemic injury) and centrally (e.g., impairing hypoxic drive to breathe). Altogether, physiological studies utilizing C93A mice are confirming the allosterically controlled role of SNO-Hb in microvascular blood flow, uncovering essential roles for RBC-mediated vasodilation in cardiovascular physiology and revealing new roles for RBCs in cardiovascular disease.
Keywords: S-nitrosothiol; S-nitrosylation; hemoglobin; microcirculation; vasodilation.
Figures
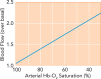
FIGURE 1.
Hypoxic autoregulation of blood flow In the classic demonstration of hypoxic autoregulation of blood flow (45), blood flow through muscle increases linearly as the oxygen saturation of the Hb entering the muscle decreases, maintaining relatively constant oxygen delivery.
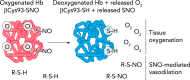
FIGURE 2.
Allosteric linkage of Hb conformation to O2 and SNO release Left: due to cooperativity, Hb in the presence of air in the lung will bind fully to four oxygen molecules (oxygenated, R-state Hb). A fraction of oxygenated Hb will also bind and stabilize SNO at Hb βCys93. Right: on reaching hypoxic tissues, Hb will cooperatively give off all four oxygen molecules during transition to the deoxygenated T-state (improving tissue oxygenation). Liberation of oxygen will cause T-state Hb βCys93-SNO to liberate SNO, which can now transfer to other cellular thiols (R-S-H), leading to vasorelaxation (SNO-mediated vasodilation).
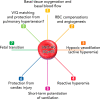
FIGURE 3.
Cardiovascular physiology regulated by βCys93 Mice lacking βCys93 have altered physiological responses due to the inability to carry and deliver SNO from Hb βCys93. These include reduced basal tissue oxygenation and blood flow (1), RBC adaptation to loss of function (2), reduced hypoxic vasodilation (active hyperemia) (3), reduced reactive hyperemia (4), absent short-term potentiation of ventilation (5), reduced resilience to cardiac injury (ischemia- and pressure overload-induced) (6), and reduced fetal transition to free-living adulthood (7). Hypothesized effects also include altered ventilation-perfusion (V/Q) matching in the lung and reduced resilience to hypoxia-driven pulmonary hypertension (8).
FIGURE 4.
_S_-nitrosothiol compensation by humanized βCys93Ala red blood cells Left: in normal RBCs and humanized Hb RBCs, SNO is carried on Hb primarily on βCys93, with minor contributions by the other Cys residues on Hb, αCys104, and βCys112. Additionally, other low molecular weight thiols and proteins, including fetal Hb γCys93, can carry SNO within RBCs (LMW-SNO; γCys93-SNO). Right: in C93A RBCs, the βCys93Ala residue (Ala93) is unable to carry SNO, but the other Hb cysteine residues show elevated SNO levels, and fetal Hb retains γCys93-SNO, which provides essential residual function (since Cys93 is invariant and βC93A mice show reduced survival). Furthermore, LMW-SNO levels are also increased. Although total RBC SNO level is essentially unchanged by C93A mutation, the distribution within RBCs and on Hb is greatly altered. Most importantly, these other Hb Cys residues carrying SNO and the LMW-SNO species do not show allosteric coupling of SNO release to hypoxia and Hb R-to-T-state transition, but instead will release SNO independent of oxygen level.
Similar articles
- Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Antioxid Redox Signal. 2021 Apr 20;34(12):936-961. doi: 10.1089/ars.2020.8153. Epub 2020 Jul 23. Antioxid Redox Signal. 2021. PMID: 32597195 Free PMC article. Review. - Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via _β_Cys93.
Hausladen A, Qian Z, Zhang R, Premont RT, Stamler JS. Hausladen A, et al. J Pharmacol Exp Ther. 2022 Jul;382(1):1-10. doi: 10.1124/jpet.122.001194. Epub 2022 May 5. J Pharmacol Exp Ther. 2022. PMID: 35512801 Free PMC article. - Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Circ Res. 2020 Jan 3;126(1):129-158. doi: 10.1161/CIRCRESAHA.119.315626. Epub 2019 Oct 8. Circ Res. 2020. PMID: 31590598 Free PMC article. Review. - SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation.
Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. Isbell TS, et al. Nat Med. 2008 Jul;14(7):773-7. doi: 10.1038/nm1771. Epub 2008 May 30. Nat Med. 2008. PMID: 18516054 Free PMC article. - Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia.
Zhang R, Hess DT, Qian Z, Hausladen A, Fonseca F, Chaube R, Reynolds JD, Stamler JS. Zhang R, et al. Proc Natl Acad Sci U S A. 2015 May 19;112(20):6425-30. doi: 10.1073/pnas.1502285112. Epub 2015 Mar 25. Proc Natl Acad Sci U S A. 2015. PMID: 25810253 Free PMC article.
Cited by
- Endothelial alpha globin is a nitrite reductase.
Keller TCS 4th, Lechauve C, Keller AS, Broseghini-Filho GB, Butcher JT, Askew Page HR, Islam A, Tan ZY, DeLalio LJ, Brooks S, Sharma P, Hong K, Xu W, Padilha AS, Ruddiman CA, Best AK, Macal E, Kim-Shapiro DB, Christ G, Yan Z, Cortese-Krott MM, Ricart K, Patel R, Bender TP, Sonkusare SK, Weiss MJ, Ackerman H, Columbus L, Isakson BE. Keller TCS 4th, et al. Nat Commun. 2022 Oct 27;13(1):6405. doi: 10.1038/s41467-022-34154-3. Nat Commun. 2022. PMID: 36302779 Free PMC article. - Hypoxic vasodilatory defect and pulmonary hypertension in mice lacking hemoglobin β-cysteine93 S-nitrosylation.
Zhang R, Hausladen A, Qian Z, Liao X, Premont RT, Stamler JS. Zhang R, et al. JCI Insight. 2022 Feb 8;7(3):e155234. doi: 10.1172/jci.insight.155234. JCI Insight. 2022. PMID: 34914637 Free PMC article. - Physiology in Perspective: The New Normal-Life in a Pandemic.
Sieck GC. Sieck GC. Physiology (Bethesda). 2020 Jul 1;35(4):220-221. doi: 10.1152/physiol.00014.2020. Physiology (Bethesda). 2020. PMID: 32490749 Free PMC article. No abstract available. - Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Antioxid Redox Signal. 2021 Apr 20;34(12):936-961. doi: 10.1089/ars.2020.8153. Epub 2020 Jul 23. Antioxid Redox Signal. 2021. PMID: 32597195 Free PMC article. Review. - Integrated pancreatic microcirculatory profiles of streptozotocin-induced and insulin-administrated type 1 diabetes mellitus.
Li Y, Li B, Wang B, Liu M, Zhang X, Li A, Zhang J, Zhang H, Xiu R. Li Y, et al. Microcirculation. 2021 Jul;28(5):e12691. doi: 10.1111/micc.12691. Epub 2021 Mar 11. Microcirculation. 2021. PMID: 33655585 Free PMC article.
References
- Bulger M, van Doorninck JH, Saitoh N, Telling A, Farrell C, Bender MA, Felsenfeld G, Axel R, Groudine M. Conservation of sequence and structure flanking the mouse and human beta-globin loci: the beta-globin genes are embedded within an array of odorant receptor genes. Proc Natl Acad Sci USA 96: 5129–5134, 1999. doi:10.1073/pnas.96.9.5129. A correction for this article is available at 10.1073/pnas.96.14.8307-a. - DOI - DOI - PMC - PubMed
- Camm AJ, Lüscher TF, Maurer G, Serruys PW (Editors). ESC CardioMed. Oxford, UK: Oxford University Press, 2018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL075443/HL/NHLBI NIH HHS/United States
- P01 HL128192/HL/NHLBI NIH HHS/United States
- R01 DK119506/DK/NIDDK NIH HHS/United States
- R01 HL126900/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources