Liquid-liquid phase separation induces pathogenic tau conformations in vitro - PubMed (original) (raw)
Liquid-liquid phase separation induces pathogenic tau conformations in vitro
Nicholas M Kanaan et al. Nat Commun. 2020.
Abstract
Formation of membrane-less organelles via liquid-liquid phase separation is one way cells meet the biological requirement for spatiotemporal regulation of cellular components and reactions. Recently, tau, a protein known for its involvement in Alzheimer's disease and other tauopathies, was found to undergo liquid-liquid phase separation making it one of several proteins associated with neurodegenerative diseases to do so. Here, we demonstrate that tau forms dynamic liquid droplets in vitro at physiological protein levels upon molecular crowding in buffers that resemble physiological conditions. Tau droplet formation is significantly enhanced by disease-associated modifications, including the AT8 phospho-epitope and the P301L tau mutation linked to an inherited tauopathy. Moreover, tau droplet dynamics are significantly reduced by these modified forms of tau. Extended phase separation promoted a time-dependent adoption of toxic conformations and oligomerization, but not filamentous aggregation. P301L tau protein showed the greatest oligomer formation following extended phase separation. These findings suggest that phase separation of tau may facilitate the formation of non-filamentous pathogenic tau conformations.
Conflict of interest statement
The authors declare no competing interests.
Figures
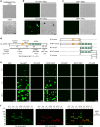
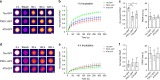
Fig. 1. Tau undergoes liquid–liquid phase separation at physiological concentrations.
a Unlabeled tau (2 μM) does not underdo LLPS without molecular crowding (−PEG), but under crowding conditions (i.e. +PEG at 10%) full-length human tau forms liquid droplets. Scale bar is 5 µm. b Similarly, tau-GFP undergoes phase separation at physiological levels (2 µM tau) to form spherical liquid droplet structures under molecular crowding conditions (+PEG), not in the absence of crowding (−PEG). Tau-GFP liquid droplets initially are formed suspended in solution (black arrowhead), and once settled on the glass slide they wet the surface (white and black arrows). Scale bars are 5 µm. c Recombinant GFP (2 µM) does not form liquid droplets whether PEG is present or not demonstrating that GFP is unlikely to drive tau-GFP LLPS. Scale bars are 5 µm. All experiments performed in LLPS buffer ±10% PEG. d Diagrams of full-length human tau protein illustrating the charges of various domains of the protein at pH 7.4 (overall protein charge is +2.7, note the NT (aa 1–224) = −11.8, and the MT (aa 225–380) = +16.3) and the CT (aa 381–441) = −2.0. The tau domain proteins used in this study also are depicted. e To study which tau domains undergo phase separation, GFP fusion proteins of specific tau domains were used, including the N terminus alone (NT, aa 1–224), the NT through the MTBRs (NTMT, aa 1–380), the MTBRs alone (MT, aa 225–380), the MTBRs through the C terminus (MTCT, aa 225–441) and the CT alone (aa 381–441). The only tau domain construct that exhibited phase separation into liquid droplets at 2 μM was the NTMT protein even after 24 h suggesting the MT and CT are not sufficient for phase separation (representative images of three independent experiments). Notably, the MT alone and MTCT proteins showed a time-dependent (primarily after 24 h) formation of small irregularly shaped structures that did not resemble liquid droplets. As expected GFP alone did not phase separate or form any structures even after 24 h incubation indicating GFP is unlikely to be responsible for the behavior of the tau proteins. f Immunoblotting of GFP and tau domain proteins incubated without (−; i.e. monomeric proteins) or with 10% PEG (+; i.e. liquid droplets) for 4 h shows a clear presence of heat-, reducing- and SDS-stable high molecular weight tau multimers only in full-length tau (tau) and NTMT tau proteins incubated with PEG. Note the other domains did not undergo liquid droplet formation and did not effectively form such multimers confirming these tau species are specifically associated with phase separation. Source data provided in the Source Data file.
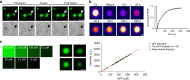
Fig. 2. Liquid droplet-like properties of phase-separated tau structures.
a Tau-GFP (2 µM) phase-separated structures undergo fusion events, which are characteristic of liquid droplets. Scale bars are 5 µm. b An illustrative example of tau-GFP fluorescence recovery after photobleaching (FRAP), which was used to confirm the dynamic nature of tau liquid droplets (see Fig. 5 for additional FRAP data). Scale bar is 1 µm. c Tau-GFP (2 µM) concentration within liquid droplets was estimated at an average of 210.7 μM (SD = ±71.82, Min = 33.9 μM, Max = 343.9 μM) after 2 h incubation (n = 50 droplets). Images of recombinant GFP protein at a range of 6–420 μM were used for a standard curve of GFP fluorescence signal (linear regression, _r_2 = 0.98). Scale bar is 5 µm. The red data points and line are the GFP standard, open black circles are the individual droplets measured and the green circle indicates the mean tau-GFP droplet intensity. Data are from a representative experiment that was repeated three times with similar results. All experiments in LLPS buffer +10% PEG. Source data for b and c provided in the Source Data file.
Fig. 3. Tau phase separates into liquid droplets in multiple buffers.
a Tau-GFP (2 µM) forms liquid droplet in the LLPS buffer, tris buffered saline, phosphate-buffered saline and X/2 buffer under crowding conditions, not in the absence of crowding. Scale bar is 5 µm. b The extent of droplet formation was significantly higher in LLPS and PBS buffers compared to X/2 buffer (graph represents mean ± SD; n = 3 independent experiments; ****p < 0.001, ***p = 0.0005, **p = 0.0098, *p = 0.0109, two-way ANOVA with Holm–Sidak post-hoc test). c Tau-GFP (2 µM) forms liquid droplets under crowding conditions (+PEG) across the range of physiological levels of ATP (0–8 mM) in the LLPS buffer. Scale bar is 5 µm. d ATP (0–8 mM) does not significantly impact the amount of tau-GFP droplet formation (graph represents mean ± SD; n = 3 independent experiments; p = 0.095, one-way ANOVA). Source data for b and d provided in the Source Data file.
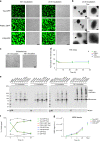
Fig. 4. Disease-related tau modifications significantly enhance liquid droplet formation.
a Images of tau-GFP, P301L-GFP, AT8-GFP liquid droplets with a range of protein concentrations (0.5–8 μM) after 2 h incubation under crowding conditions. Scale bar is 40 µm. b Linear regression analysis for estimating the critical droplet concentration (i.e. _x_-intercept) for each tau construct (graph represents mean ± SD; n = 3 independent experiments). The CDC for P301L-GFP (0.53 μM; _r_2 = 0.99) and AT8-GFP (0.63 μM; _r_2 = 0.98) were slightly lower than the CDC of tau-GFP (0.74 μM; _r_2 = 0.98), but the differences did not reach statistical significance (p = 0.32, one-way ANOVA). c Images of tau-GFP, P301L-GFP and AT8-GFP (all at 2 µM) liquid droplets under crowding conditions after 1 h incubation. Scale bar is 2 μm. d The extent of droplet formation (total area), individual droplet size and droplet fluorescence intensity were significantly higher in P301L-GFP and AT8-GFP droplets compared to tau-GFP droplets (graph represents mean ± SD; n = 3 independent experiments; one-way ANOVA with Holm–Sidak post-hoc test, ****p = 0.0039, ***p = 0.0075, **p = 0.0021, *p = 0.0272, #p = 0.0474). All experiments in LLPS buffer +10% PEG. Source data for b and d provided in the Source Data file.
Fig. 5. Disease-related tau modifications form liquid droplet with reduced dynamic properties.
a Representative droplets of tau-GFP, P301L-GFP and AT8-GFP (4 µM each, 2 µM tau-GFP and 2 µM tau unlabeled) analyzed using FRAP after 1 h incubation in crowding conditions. b FRAP curves for each tau protein after 1 h incubation indicate that each protein shows dynamic exchange of proteins with the surrounding bulk phase (graph represents mean ± SEM). c Comparison of the final extent of recovery after photobleaching (i.e. 320 s) demonstrates that tau-GFP liquid droplets show significantly more recovery than P301L-GFP and AT8-GFP droplets after 1 h incubation (one-way ANOVA with Holm–Sidak post-hoc test, **p = 0.0054, *p = 0.0237, graph represents mean ± SD). The FRAP time was not statistically different between tau constructs (p = 0.24, one-way ANOVA). d Representative droplets of tau-GFP, P301L-GFP and AT8-GFP (4 µM each, 2 µM tau-GFP and 2 µM tau unlabeled) analyzed using FRAP after 4 h incubation in crowding conditions. e FRAP curves for each tau protein analyzed after 4 h incubation demonstrate marked reductions in the dynamic properties of droplets from all three tau proteins (graph represents mean ± SEM). f Comparison of the final extent of recovery after photobleaching (i.e. 320 s) for all tau liquid droplets show similar low levels of recovery after 4 h incubation (p = 0.30, one-way ANOVA, graph represents mean ± SD). The FRAP time was not statistically different between tau constructs (p = 0.31, one-way ANOVA). The 1 and 4 h FRAP experiments were repeated five independent times. Source data for b, c, e and f provided in the Source Data file.
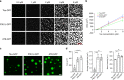
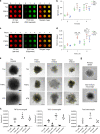
Fig. 6. Prolonged incubation of tau liquid droplets produces multimeric tau species but does not lead to fibril formation.
a Images of phase-separated structures formed by tau-GFP, P301L-GFP and AT8-GFP (each at 4 µM) after 16 and 24 h incubation at room temperature. Scale bar is 5 µm. b Transmission electron micrographs of phase-separated structures from unlabeled tau, P301L and AT8 proteins (4 µM each) after 24 h incubation at room temperature. Tau filaments were not readily apparent in the structures formed under these conditions (arrows indicate the droplet shown in the higher magnification images). Scale bars are 800 nm (left images) and 400 nm (right images). This experiment was repeated three independent times. c Incubation of unlabeled tau for 24 and 48 h produces droplets, not fibrillar structures, further confirming the lack of fibril formation via LLPS. d Thioflavin S (ThS), a fibrillar cross-β-sheet binding dye that labels tau filaments, showed low signal and no increase over time (1–48 h, data normalized to LLPS buffer with PEG blank) with prolonged phase separation confirming the lack of cross-β-sheet structures in tau proteins (e.g. filaments) (graph represents mean ± SD). Note that the positive control samples, arachidonic acid-induced tau aggregates (black circle), showed a robust increase in ThS intensity. e Supernatant (Sup) and pelleted (Pell) fractions from droplet spin down experiments were run in western blots to assess the time-dependent (0–24 h of phase separation) formation of heat-, reducing- and SDS-stable tau multimers. Notably, some high molecular weight tau species (HMW, i.e. tau multimers) were apparent shortly after addition of PEG (0 h time point). f Quantification of total tau in the Sup or Pell fractions shows a clear time-dependent shift in pelletable tau starting 1 h after phase separation is initiated and continuing through 24 h (graph represents mean ± SD). g Quantification of the HMW tau multimers shows a time-dependent increase in stable multimers with prolonged phase separation with all tau proteins (graph represents mean ± SD). Source data for d–g provided in the Source Data file.
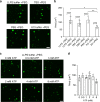
Fig. 7. Phase separation leads to formation of pathological tau conformations and oligomeric tau.
a Unlabeled tau, P301L and AT8 tau proteins incubated in LLPS buffer for 0, 1 or 4 h (each at 4 µM) were dot blotted and probed for total tau (R1 antibody) and PAD-exposed tau (TNT2 antibody). b Quantitation of dot blots show significant increases in PAD exposure upon incubation of tau, P301L and AT8 proteins with PEG for 1 and 4 h compared to 0 h (n = 3 independent experiments; two-way ANOVA with Sidak post-hoc test ***p < 0.0001, **p = 0.0001, *p = 0.0002 compared to respective 0 h time point; graphs are mean ± SD). c Representative dot blot images of unlabeled tau, P301L and AT8 tau proteins incubated for 0, 1 or 4 h in LLPS buffer and probed for total tau (R1 antibody) and oligomeric tau (TOC1 antibody). d Quantitation of dot blots show significant time-dependent increases in oligomeric tau species upon LLPS of tau, P301L and AT8 with significantly more TOC1 reactivity in the P301L and AT8 samples compared to tau (n = 3 independent experiments; two-way ANOVA with Sidak post-hoc test, ****p < 0.0001, ***p = 0.0003, **p = 0.0402, compared to respective 0 h time point, *p = 0.0420 compared to respective 0 and 4 h time point, ####p < 0.0001, ###p = 0.0017, ##p = 0.0095, #p = 0.0328; graphs are mean ± SD). e Transmission electron micrographs of negatively stained liquid droplets composed of unlabeled tau, P301L tau and AT8 tau (4 µM, 4 h incubation). Scale bars are 200 nm. f Immunogold labeling of tau, P301L and AT8 liquid droplets (4 µM, 4 h incubation) confirm the presence of PAD-exposed tau (TNT2 antibody) and oligomeric tau (TOC1 antibody) within droplets. A pan-tau antibody (Tau5) confirmed the structures are composed of tau. Yellow circles are around gold particles for easier visualization and scale bars are 200 nm. g Primary antibody delete and non-immune mouse IgG (substituted as the primary antibody) controls confirm the specificity of the immunogold labeling in f. Scale bars are 200 nm. h Quantitation of gold particle density demonstrates a clear increase in labeling (~10-fold) with each antibody within droplet when compared to the grid, confirming the specificity for labeling within droplets (graph represents mean ± SD). Source data for a–d, h provided in the Source Data file.
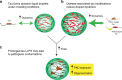
Fig. 8. Proposed hypothetical model of tau liquid–liquid phase separation in normal and pathological conditions.
a Under normal physiological conditions, phase separation could lead to the formation of tau liquid droplet-like structures that show high dynamics. b Forms of tau associated with disease, such as phosphorylated tau (e.g. at the AT8 site) or mutant tau that causes inherited tauopathies, facilitate liquid droplet formation. c Pathological conditions that facilitate tau phase separation and/or prolong phase separation may lead to the adoption of pathological tau conformations (i.e. PAD exposure and oligomerization) that have been linked to cell toxicity.
Similar articles
- Tau protein liquid-liquid phase separation can initiate tau aggregation.
Wegmann S, Eftekharzadeh B, Tepper K, Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D, Kamath T, Commins C, Vanderburg C, Roe AD, Fan Z, Molliex AM, Hernandez-Vega A, Muller D, Hyman AA, Mandelkow E, Taylor JP, Hyman BT. Wegmann S, et al. EMBO J. 2018 Apr 3;37(7):e98049. doi: 10.15252/embj.201798049. Epub 2018 Feb 22. EMBO J. 2018. PMID: 29472250 Free PMC article. - Tau liquid-liquid phase separation: At the crossroads of tau physiology and tauopathy.
Islam M, Shen F, Regmi D, Petersen K, Karim MRU, Du D. Islam M, et al. J Cell Physiol. 2024 Jun;239(6):e30853. doi: 10.1002/jcp.30853. Epub 2022 Aug 18. J Cell Physiol. 2024. PMID: 35980344 Free PMC article. Review. - Regulatory mechanisms of tau protein fibrillation under the conditions of liquid-liquid phase separation.
Boyko S, Surewicz K, Surewicz WK. Boyko S, et al. Proc Natl Acad Sci U S A. 2020 Dec 15;117(50):31882-31890. doi: 10.1073/pnas.2012460117. Epub 2020 Dec 1. Proc Natl Acad Sci U S A. 2020. PMID: 33262278 Free PMC article. - Zinc promotes liquid-liquid phase separation of tau protein.
Singh V, Xu L, Boyko S, Surewicz K, Surewicz WK. Singh V, et al. J Biol Chem. 2020 May 1;295(18):5850-5856. doi: 10.1074/jbc.AC120.013166. Epub 2020 Mar 30. J Biol Chem. 2020. PMID: 32229582 Free PMC article. - Liquid-liquid phase separation of protein tau: An emerging process in Alzheimer's disease pathogenesis.
Ainani H, Bouchmaa N, Ben Mrid R, El Fatimy R. Ainani H, et al. Neurobiol Dis. 2023 Mar;178:106011. doi: 10.1016/j.nbd.2023.106011. Epub 2023 Jan 23. Neurobiol Dis. 2023. PMID: 36702317 Review.
Cited by
- The structure and phase of tau: from monomer to amyloid filament.
Zeng Y, Yang J, Zhang B, Gao M, Su Z, Huang Y. Zeng Y, et al. Cell Mol Life Sci. 2021 Mar;78(5):1873-1886. doi: 10.1007/s00018-020-03681-x. Epub 2020 Oct 19. Cell Mol Life Sci. 2021. PMID: 33078207 Free PMC article. Review. - Study of Tau Liquid-Liquid Phase Separation In Vitro.
Boyko S, Surewicz WK. Boyko S, et al. Methods Mol Biol. 2023;2551:245-252. doi: 10.1007/978-1-0716-2597-2_16. Methods Mol Biol. 2023. PMID: 36310207 - Aggregation of the amyloid-β peptide (Aβ40) within condensates generated through liquid-liquid phase separation.
Morris OM, Toprakcioglu Z, Röntgen A, Cali M, Knowles TPJ, Vendruscolo M. Morris OM, et al. Sci Rep. 2024 Sep 30;14(1):22633. doi: 10.1038/s41598-024-72265-7. Sci Rep. 2024. PMID: 39349560 Free PMC article. - Single-molecule imaging reveals Tau trapping at nanometer-sized dynamic hot spots near the plasma membrane that persists after microtubule perturbation and cholesterol depletion.
Padmanabhan P, Kneynsberg A, Cruz E, Amor R, Sibarita JB, Götz J. Padmanabhan P, et al. EMBO J. 2022 Oct 4;41(19):e111265. doi: 10.15252/embj.2022111265. Epub 2022 Aug 25. EMBO J. 2022. PMID: 36004506 Free PMC article. - Neuronal biomolecular condensates and their implications in neurodegenerative diseases.
Nam J, Gwon Y. Nam J, et al. Front Aging Neurosci. 2023 Mar 24;15:1145420. doi: 10.3389/fnagi.2023.1145420. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37065458 Free PMC article. Review.
References
- Hyman AA, Weber CA, Julicher F. Liquid-liquid phase separation in biology. Annu Rev. Cell Dev. Biol. 2014;30:39–58. - PubMed
- Souquere S, et al. Unravelling the ultrastructure of stress granules and associated P-bodies in human cells. J. Cell Sci. 2009;122:3619–3626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources