Regulation of Epithelial-to-Mesenchymal Transition by Alternative Translation Initiation Mechanisms and Its Implications for Cancer Metastasis - PubMed (original) (raw)
Review
Regulation of Epithelial-to-Mesenchymal Transition by Alternative Translation Initiation Mechanisms and Its Implications for Cancer Metastasis
Amit Bera et al. Int J Mol Sci. 2020.
Abstract
Translation initiation plays a critical role in the regulation of gene expression for development and disease conditions. During the processes of development and disease, cells select specific mRNAs to be translated by controlling the use of diverse translation initiation mechanisms. Cells often switch translation initiation from a cap-dependent to a cap-independent mechanism during epithelial-to-mesenchymal transition (EMT), a process that plays an important role in both development and disease. EMT is involved in tumor metastasis because it leads to cancer cell migration and invasion, and is also associated with chemoresistance. In this review we will provide an overview of both the internal ribosome entry site (IRES)-dependent and N6-methyladenosine (m6A)-mediated translation initiation mechanisms and discuss how cap-independent translation enables cells from primary epithelial tumors to achieve a motile mesenchymal-like phenotype, which in turn drives tumor metastasis.
Keywords: IRES; ITAF; cancer; epithelial-to-mesenchymal transition (EMT); m6A-mediated translation; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
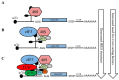
Overview of internal ribosome entry site (IRES)-mediated translation initiation. The requirement for canonical initiation factors and IRES trans-acting factors (ITAFs) varies among IRES-containing mRNAs. Several types of IRES-mediated translation initiation have been identified: (A) Group I: no canonical factors are required and the 40S ribosome is directly recruited to the mRNA by the IRES structure; (B) Group II: a few canonical initiation factors are required for 40S ribosome recruitment; (C) Groups III and IV: multiple canonical initiation factors, as well as ITAFs, are required for 40S ribosome recruitment and translation initiation.
Figure 2
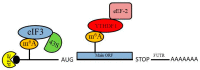
Overview of the role of N6-methyladenosine (m6A) modification of mRNA in protein synthesis. m6A modification of mRNA within the 5ʹ untranslated region (5ʹ-UTR) permits direct binding of the eIF3 complex to facilitate recruitment of the 43S pre-initiation complex, which mediates translation initiation. m6A modification within the coding sequence can enhance recruitment of the eEF-2 elongation factor via YDHTF1 binding to the m6A residue, which enhances translation of the mRNA.
Figure 3
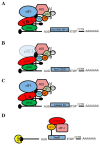
Alternative translation mechanisms are involved in the synthesis of proteins with roles in epithelial-to-mesenchymal transition (EMT). (A) The YB-1 protein recruits the translation initiation machinery to mRNAs that encode Snail1, ZEB2, and HIF-1α to support their synthesis, leading to EMT. (B) Reduced expression of the initiation factor eIF3e facilitates the translation of the Snail1 and ZEB2 proteins to mediate EMT. (C) The ITAF La mediates the translation of Laminin B1 to drive cells toward EMT. (D) m6A modifications within the Snail1 open reading frame permit enhanced recruitment of the elongation factor eEF-2 via YTHDF1 to increase Snail 1 translation and facilitate EMT.
Similar articles
- Breast cancer cell mesenchymal transition and metastasis directed by DAP5/eIF3d-mediated selective mRNA translation.
Alard A, Katsara O, Rios-Fuller T, Parra C, Ozerdem U, Ernlund A, Schneider RJ. Alard A, et al. Cell Rep. 2023 Jun 27;42(6):112646. doi: 10.1016/j.celrep.2023.112646. Epub 2023 Jun 13. Cell Rep. 2023. PMID: 37314929 Free PMC article. - On translational regulation and EMT.
Evdokimova V, Tognon CE, Sorensen PH. Evdokimova V, et al. Semin Cancer Biol. 2012 Oct;22(5-6):437-45. doi: 10.1016/j.semcancer.2012.04.007. Epub 2012 Apr 23. Semin Cancer Biol. 2012. PMID: 22554796 Review. - EMT Transition States during Tumor Progression and Metastasis.
Pastushenko I, Blanpain C. Pastushenko I, et al. Trends Cell Biol. 2019 Mar;29(3):212-226. doi: 10.1016/j.tcb.2018.12.001. Epub 2018 Dec 26. Trends Cell Biol. 2019. PMID: 30594349 Review. - Tumor microenvironment and noncoding RNAs as co-drivers of epithelial-mesenchymal transition and cancer metastasis.
Drak Alsibai K, Meseure D. Drak Alsibai K, et al. Dev Dyn. 2018 Mar;247(3):405-431. doi: 10.1002/dvdy.24548. Epub 2017 Sep 18. Dev Dyn. 2018. PMID: 28691356 Review. - MicroRNAs: regulators of cancer metastasis and epithelial-mesenchymal transition (EMT).
Ding XM. Ding XM. Chin J Cancer. 2014 Mar;33(3):140-7. doi: 10.5732/cjc.013.10094. Epub 2013 Sep 9. Chin J Cancer. 2014. PMID: 24016392 Free PMC article. Review.
Cited by
- Therapeutic targeting approach on epithelial-mesenchymal plasticity to combat cancer metastasis.
Mishra AB, Nishank SS. Mishra AB, et al. Med Oncol. 2023 May 29;40(7):190. doi: 10.1007/s12032-023-02049-y. Med Oncol. 2023. PMID: 37247000 Review. - Non-canonical mRNA translation initiation in cell stress and cancer.
Mahé M, Rios-Fuller T, Katsara O, Schneider RJ. Mahé M, et al. NAR Cancer. 2024 May 31;6(2):zcae026. doi: 10.1093/narcan/zcae026. eCollection 2024 Jun. NAR Cancer. 2024. PMID: 38828390 Free PMC article. Review. - Breast cancer cell mesenchymal transition and metastasis directed by DAP5/eIF3d-mediated selective mRNA translation.
Alard A, Katsara O, Rios-Fuller T, Parra C, Ozerdem U, Ernlund A, Schneider RJ. Alard A, et al. Cell Rep. 2023 Jun 27;42(6):112646. doi: 10.1016/j.celrep.2023.112646. Epub 2023 Jun 13. Cell Rep. 2023. PMID: 37314929 Free PMC article. - Upregulation of CPNE7 in mesenchymal stromal cells promotes oral squamous cell carcinoma metastasis through the NF-κB pathway.
Ji X, Sun T, Xie S, Qian H, Song L, Wang L, Liu H, Feng Q. Ji X, et al. Cell Death Discov. 2021 Oct 14;7(1):294. doi: 10.1038/s41420-021-00684-w. Cell Death Discov. 2021. PMID: 34650058 Free PMC article. - Regulating epithelial-mesenchymal plasticity from 3D genome organization.
Pang QY, Chiu YC, Huang RY. Pang QY, et al. Commun Biol. 2024 Jun 20;7(1):750. doi: 10.1038/s42003-024-06441-w. Commun Biol. 2024. PMID: 38902393 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous