Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study - PubMed (original) (raw)
Multicenter Study
. 2020 Oct;20(10):1135-1140.
doi: 10.1016/S1473-3099(20)30434-5. Epub 2020 Jun 8.
Aurelio Sonzogni 1, Ahmed Nasr 2, Roberta Simona Rossi 1, Alessandro Pellegrinelli 1, Pietro Zerbi 3, Roberto Rech 4, Riccardo Colombo 4, Spinello Antinori 5, Mario Corbellino 6, Massimo Galli 5, Emanuele Catena 4, Antonella Tosoni 1, Andrea Gianatti 1, Manuela Nebuloni 7
Affiliations
- PMID: 32526193
- PMCID: PMC7279758
- DOI: 10.1016/S1473-3099(20)30434-5
Multicenter Study
Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study
Luca Carsana et al. Lancet Infect Dis. 2020 Oct.
Abstract
Background: COVID-19 is characterised by respiratory symptoms, which deteriorate into respiratory failure in a substantial proportion of cases, requiring intensive care in up to a third of patients admitted to hospital. Analysis of the pathological features in the lung tissues of patients who have died with COVID-19 could help us to understand the disease pathogenesis and clinical outcomes.
Methods: We systematically analysed lung tissue samples from 38 patients who died from COVID-19 in two hospitals in northern Italy between Feb 29 and March 24, 2020. The most representative areas identified at macroscopic examination were selected, and tissue blocks (median seven, range five to nine) were taken from each lung and fixed in 10% buffered formalin for at least 48 h. Tissues were assessed with use of haematoxylin and eosin staining, immunohistochemical staining for inflammatory infiltrate and cellular components (including staining with antibodies against CD68, CD3, CD45, CD61, TTF1, p40, and Ki-67), and electron microscopy to identify virion localisation.
Findings: All cases showed features of the exudative and proliferative phases of diffuse alveolar damage, which included capillary congestion (in all cases), necrosis of pneumocytes (in all cases), hyaline membranes (in 33 cases), interstitial and intra-alveolar oedema (in 37 cases), type 2 pneumocyte hyperplasia (in all cases), squamous metaplasia with atypia (in 21 cases), and platelet-fibrin thrombi (in 33 cases). The inflammatory infiltrate, observed in all cases, was largely composed of macrophages in the alveolar lumina (in 24 cases) and lymphocytes in the interstitium (in 31 cases). Electron microscopy revealed that viral particles were predominantly located in the pneumocytes.
Interpretation: The predominant pattern of lung lesions in patients with COVID-19 patients is diffuse alveolar damage, as described in patients infected with severe acute respiratory syndrome and Middle East respiratory syndrome coronaviruses. Hyaline membrane formation and pneumocyte atypical hyperplasia are frequent. Importantly, the presence of platelet-fibrin thrombi in small arterial vessels is consistent with coagulopathy, which appears to be common in patients with COVID-19 and should be one of the main targets of therapy.
Funding: None.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
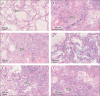
Figure 1
Haematoxylin and eosin-stained sections from representative areas of lung parenchyma with diffuse alveolar damage (A) Exudative phase of diffuse alveolar damage with hyaline membranes (arrow). (B) Organising microthrombus (arrow). (C) Concomitant interstitial pneumonia, intra-alveolar scattered multinucleated giant cells (top, left), and outstanding epithelial proliferation around a bronchiole with plurifocal squamous differentiation and mild atypia (arrow). (D) Early proliferative phase of diffuse alveolar damage with many hyperplastic, and rarely atypical, type 2 pneumocytes. (E) Intermediate phase of diffuse alveolar damage with initial organising aspects (arrow) and interstitial pneumonia with marked lymphocytic infiltrate. (F) Advanced proliferative phase of diffuse alveolar damage with interstitial myofibroblastic reaction, diffuse lymphocytic interstitial infiltrate, and residual scattered hyperplastic type 2 pneumocytes (arrow). (A, D, E) Original magnification × 20. (B, C, F) Original magnification × 10.
Figure 2
Electron microscopy of a representative case Flat type 2 pneumocyte without lamellar electron-dense bodies of surfactant free in the alveolar space, containing numerous virions (inset bottom right) in cytoplasmic vacuoles (arrow) and along the plasma membrane (arrow heads). Virions had an average diameter of 82 nm, and viral projection about 13 nm in length (inset upper left, original magnification × 85 000).
Comment in
- Pathologists in pursuit of the COVID-19 culprit.
Yi ES, Cecchini MJ, Bois MC. Yi ES, et al. Lancet Infect Dis. 2020 Oct;20(10):1102-1103. doi: 10.1016/S1473-3099(20)30449-7. Epub 2020 Jun 8. Lancet Infect Dis. 2020. PMID: 32526191 Free PMC article. No abstract available. - Lung fibrosis: an undervalued finding in COVID-19 pathological series.
Grillo F, Barisione E, Ball L, Mastracci L, Fiocca R. Grillo F, et al. Lancet Infect Dis. 2021 Apr;21(4):e72. doi: 10.1016/S1473-3099(20)30582-X. Epub 2020 Jul 28. Lancet Infect Dis. 2021. PMID: 32735785 Free PMC article. No abstract available. - The pathogenesis of thromboembolic disease in covid-19 patients: Could be a catastrophic antiphospholipid syndrome?
Previtali G, Seghezzi M, Moioli V, Sonzogni A, Cerutti L, Marozzi R, Ravasio R, Gianatti A, Guerra G, Alessio MG. Previtali G, et al. Thromb Res. 2020 Oct;194:192-194. doi: 10.1016/j.thromres.2020.06.042. Epub 2020 Jun 27. Thromb Res. 2020. PMID: 32788116 Free PMC article. No abstract available.
Similar articles
- COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City.
Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, Mostyka M, Elsoukkary S, He B, Del Vecchio C, Fortarezza F, Pezzuto F, Navalesi P, Crisanti A, Fowkes ME, Bryce CH, Calabrese F, Beasley MB. Borczuk AC, et al. Mod Pathol. 2020 Nov;33(11):2156-2168. doi: 10.1038/s41379-020-00661-1. Epub 2020 Sep 2. Mod Pathol. 2020. PMID: 32879413 Free PMC article. - [A pathological report of three COVID-19 cases by minimal invasive autopsies].
Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. Yao XH, et al. Zhonghua Bing Li Xue Za Zhi. 2020 May 8;49(5):411-417. doi: 10.3760/cma.j.cn112151-20200312-00193. Zhonghua Bing Li Xue Za Zhi. 2020. PMID: 32172546 Chinese. - Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series.
Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Bradley BT, et al. Lancet. 2020 Aug 1;396(10247):320-332. doi: 10.1016/S0140-6736(20)31305-2. Epub 2020 Jul 16. Lancet. 2020. PMID: 32682491 Free PMC article. - COVID-19: Brief check through the pathologist's eye (autopsy archive).
Mansueto G. Mansueto G. Pathol Res Pract. 2020 Nov;216(11):153195. doi: 10.1016/j.prp.2020.153195. Epub 2020 Aug 28. Pathol Res Pract. 2020. PMID: 32890939 Free PMC article. Review. - Pulmonary pathology and COVID-19: lessons from autopsy. The experience of European Pulmonary Pathologists.
Calabrese F, Pezzuto F, Fortarezza F, Hofman P, Kern I, Panizo A, von der Thüsen J, Timofeev S, Gorkiewicz G, Lunardi F. Calabrese F, et al. Virchows Arch. 2020 Sep;477(3):359-372. doi: 10.1007/s00428-020-02886-6. Epub 2020 Jul 9. Virchows Arch. 2020. PMID: 32642842 Free PMC article. Review.
Cited by
- Six and twelve-month respiratory outcomes in a cohort of severe and critical COVID-19 survivors: A prospective monocentric study in Latin America.
Fernández-Trujillo L, Galindo-Sánchez JS, Cediel A, García CA, Morales EI, Largo J, Amezquita-Dussan MA. Fernández-Trujillo L, et al. SAGE Open Med. 2024 Sep 9;12:20503121241275369. doi: 10.1177/20503121241275369. eCollection 2024. SAGE Open Med. 2024. PMID: 39263637 Free PMC article. - Phenotypic changes in immune cells induced by granulocyte and monocyte adsorptive apheresis in patients with severe COVID-19: An ex vivo study.
Hisamune R, Yamakawa K, Kayano K, Ushio N, Wada T, Taniguchi K, Takasu A. Hisamune R, et al. Acute Med Surg. 2024 Aug 29;11(1):e70003. doi: 10.1002/ams2.70003. eCollection 2024 Jan-Dec. Acute Med Surg. 2024. PMID: 39211524 Free PMC article. - The pathological maelstrom of COVID-19 and cardiovascular disease.
Giacca M, Shah AM. Giacca M, et al. Nat Cardiovasc Res. 2022 Mar;1(3):200-210. doi: 10.1038/s44161-022-00029-5. Epub 2022 Mar 16. Nat Cardiovasc Res. 2022. PMID: 39195986 Review. - A follow-up study of post-COVID-19 syndrome in hospitalized children with Omicron variant infection in Wuhan.
Tang C, Wang S, Fan J. Tang C, et al. Front Pediatr. 2024 Aug 1;12:1359057. doi: 10.3389/fped.2024.1359057. eCollection 2024. Front Pediatr. 2024. PMID: 39149538 Free PMC article. - Extended Prone Position and 90-Day Mortality in Mechanically Ventilated Patients With COVID-19.
Estrella-Alonso A, Silva-Obregón JA, Fernández-Tobar R, Marián-Crespo C, Ruiz de Santaquiteria-Torres V, Jiménez-Puente G, Arroyo-Espliguero R, Viana-Llamas MC, Ramírez-Cervantes KL, Quintana-Díaz M. Estrella-Alonso A, et al. Respir Care. 2024 Sep 26;69(10):1255-1265. doi: 10.4187/respcare.11622. Respir Care. 2024. PMID: 39137953
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous