From threat to cure: understanding of virus-induced cell death leads to highly immunogenic oncolytic influenza viruses - PubMed (original) (raw)
Review
From threat to cure: understanding of virus-induced cell death leads to highly immunogenic oncolytic influenza viruses
Julijan Kabiljo et al. Cell Death Discov. 2020.
Abstract
Oncolytic viruses constitute an emerging strategy in immunomodulatory cancer treatment. The first oncolytic virus, Talimogene laherparepvec (T-VEC), based on herpes simplex virus 1 (HSV-1), was approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2015. The field of oncolytic virotherapy is still in its beginnings, since many promising viruses remain only superficially explored. Influenza A virus causes a highly immunogenic acute infection but never leads to a chronic disease. While oncolytic influenza A viruses are in preclinical development, they have not made the transition into clinical practice yet. Recent insights into different types of cell death caused by influenza A virus infection illuminate novel possibilities of enhancing its therapeutic effect. Genetic engineering and experience in influenza A virus vaccine development allow safe application of the virus in patients. In this review we give a summary of efforts undertaken to develop oncolytic influenza A viruses. We discuss strategies for targeting viral replication to cancerous lesions and arming them with immunogenic transgenes. We furthermore describe which modes of cell death are induced by influenza A virus infection and how these insights may be utilized to optimize influenza A virus-based oncolytic virus design.
Keywords: Cancer immunotherapy; Tumour immunology; Tumour virus infections.
© The Author(s) 2020.
Conflict of interest statement
Conflict of interestM.B. is a cofounder of Vacthera, a company seeking to develop oncolytic influenza viruses. M.B. and J.L. received an investigator-sponsored research grant from Bristol-Myers-Squibb in the field of tumor immunology. M.B. received consultant fees from Bristol-Myers-Squibb.
Figures
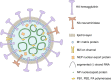
Fig. 1. Components of the influenza A virus.
Schematic representation of all components of the influenza A virus virion.
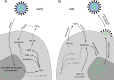
Fig. 2. A proposed model of how influenza A virus controls apoptosis in 2 phases.
a In the early phase of an influenza virus infection, the virus benefits from reduced apoptosis, in order to transcribe viral structural proteins and replicate viral RNA. Anti-apoptotic functions are mediated by NS1, interfering with various danger signaling proteins and reducing NF-κB induced TNFα release. b In the late phase of influenza virus infection, the pro apoptotic viral protein PB1-F2 and the weakened TNFα release might accumulate, leading to enhanced apoptotic signaling. Apoptosis enhances the release of viral RNA from the nucleus, a prerequisite for virus assembly and release. NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; TNFα, tumor necrosis factor alpha.
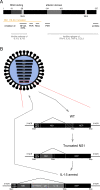
Fig. 3. Functions of the influenza A virus NS1 protein and generation of an armed oncolytic influenza A virus.
a Schematic representation of the NS1 protein. Major domains are represented and their AA positions indicated above. The yellow line represents parts of NS1 expressed after truncation to 116 AA. Inhibitory functions of NS1 relevant to oncolytic virus development and the domains they have been attributed to are indicated,,,,,,. b Example of genetic modifications in an influenza virus designed to be used as oncolytic agent. Modifications are carried out on the NS segment. In the first step NS1 is truncated to a length of 116 AA, leaving NEP intact. In a second step the armed transgene, in this example IL-15, is encoded in the reading frame of NS1. It is connected to an IgK and separated from NS1 with the H2 FMDV. Reading frames for NS1 and NEP have a common beginning. NS1 continues on, while an alternative reading frame is created for NEP through splicing. The short, frame shifted overlap of the end of NS1 and middle part of NEP is indicated by the diagonal hatching pattern. The complete NEP reading frame is generated through splicing in the attenuated and armed examples as well. AA amino acids, IL-15 Interleukin-15, NS1 nonstructural 1 protein, NEP nuclear export protein, IgK mouse-derived IgKappa signal peptide, 2A FMDV 2A cleavage site of the foot and mouth disease virus.
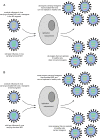
Fig. 4. Reassortment of oncolytic influenza A viruses and wild-type influenza A viruses.
Examples of reassortment of an armed oncolytic influenza virus attenuated by NS1 truncation during co-infection with wild-type influenza virus. a represents transgene expression from the NS1 reading frame. All reassortants carrying the transgene are attenuated. b represents transgene expression from the M segment. Some reassortants may carry both wild-type elements of the attenuation marker NS1 and a transgene, leading to unpredictable effects of the newly created virus. NS1 nonstructural 1 protein.
Similar articles
- The Oncolytic Herpes Simplex Virus Talimogene Laherparepvec Shows Promising Efficacy in Neuroendocrine Cancer Cell Lines.
Kloker LD, Berchtold S, Smirnow I, Schaller M, Fehrenbacher B, Krieg A, Sipos B, Lauer UM. Kloker LD, et al. Neuroendocrinology. 2019;109(4):346-361. doi: 10.1159/000500159. Epub 2019 Jun 13. Neuroendocrinology. 2019. PMID: 31280274 - Comparison of the oncolytic activity of a replication-competent and a replication-deficient herpes simplex virus 1.
Lindner G, Walter A, Magnus CL, Rosenhammer K, Holoborodko B, Koch V, Hirsch S, Grossmann L, Li S, Knipe DM, DeLuca N, Schuler-Thurner B, Gross S, Schwertner B, Toelge M, Rohrhofer A, Stöckl S, Bauer RJ, Knoll G, Ehrenschwender M, Haferkamp S, Schmidt B, Schuster P. Lindner G, et al. Immunology. 2024 Jun;172(2):279-294. doi: 10.1111/imm.13775. Epub 2024 Mar 5. Immunology. 2024. PMID: 38444199 - Talimogene Laherparepvec (T-VEC) and Other Oncolytic Viruses for the Treatment of Melanoma.
Bommareddy PK, Patel A, Hossain S, Kaufman HL. Bommareddy PK, et al. Am J Clin Dermatol. 2017 Feb;18(1):1-15. doi: 10.1007/s40257-016-0238-9. Am J Clin Dermatol. 2017. PMID: 27988837 Free PMC article. Review. - Novel Oncolytic Herpes Simplex Virus 1 VC2 Promotes Long-Lasting, Systemic Anti-melanoma Tumor Immune Responses and Increased Survival in an Immunocompetent B16F10-Derived Mouse Melanoma Model.
Uche IK, Fowlkes N, Vu L, Watanabe T, Carossino M, Nabi R, Del Piero F, Rudd JS, Kousoulas KG, Rider PJF. Uche IK, et al. J Virol. 2021 Jan 13;95(3):e01359-20. doi: 10.1128/JVI.01359-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177208 Free PMC article. - Intratumoral Immunotherapy-Update 2019.
Hamid O, Ismail R, Puzanov I. Hamid O, et al. Oncologist. 2020 Mar;25(3):e423-e438. doi: 10.1634/theoncologist.2019-0438. Epub 2019 Nov 29. Oncologist. 2020. PMID: 32162802 Free PMC article. Review.
Cited by
- Cancer-associated fibroblasts shape early myeloid cell response to chemotherapy-induced immunogenic signals in next generation tumor organoid cultures.
Kabiljo J, Theophil A, Homola J, Renner AF, Stürzenbecher N, Ammon D, Zirnbauer R, Stang S, Tran L, Laengle J, Kulu A, Chen A, Fabits M, Atanasova VS, Pusch O, Weninger W, Walczak H, Herndler Brandstetter D, Egger G, Dolznig H, Kusienicka A, Farlik M, Bergmann M. Kabiljo J, et al. J Immunother Cancer. 2024 Nov 4;12(11):e009494. doi: 10.1136/jitc-2024-009494. J Immunother Cancer. 2024. PMID: 39500527 Free PMC article. - Oncolytic virotherapy for hepatocellular carcinoma: A potent immunotherapeutic landscape.
Xiao R, Jin H, Huang F, Huang B, Wang H, Wang YG. Xiao R, et al. World J Gastrointest Oncol. 2024 Jul 15;16(7):2867-2876. doi: 10.4251/wjgo.v16.i7.2867. World J Gastrointest Oncol. 2024. PMID: 39072175 Free PMC article. - Ferroptosis in viral infection: a potential therapeutic target.
Ding L. Ding L. Future Microbiol. 2024;19(6):519-524. doi: 10.2217/fmb-2023-0186. Epub 2024 Feb 27. Future Microbiol. 2024. PMID: 38411103 Review. - Plant extracts modulate cellular stress to inhibit replication of mouse Coronavirus MHV-A59.
Prieto K, Arévalo C, Lasso P, Carlosama C, Urueña C, Fiorentino S, Barreto A. Prieto K, et al. Heliyon. 2023 Dec 8;10(1):e23403. doi: 10.1016/j.heliyon.2023.e23403. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38169850 Free PMC article. - Immune responses elicited by ssRNA(-) oncolytic viruses in the host and in the tumor microenvironment.
Bykov Y, Dawodu G, Javaheri A, Garcia-Sastre A, Cuadrado-Castano S. Bykov Y, et al. J Cancer Metastasis Treat. 2023;9:10. doi: 10.20517/2394-4722.2022.92. Epub 2023 Apr 4. J Cancer Metastasis Treat. 2023. PMID: 37974615 Free PMC article.
References
- Bluming AZ, Ziegler JL. Regression of Burkitt’s lymphoma in association with measles infection. Lancet (Lond., Engl.) 1971;2:105–106. - PubMed
- Taqi AM, Abdurrahman MB, Yakubu AM, Fleming AF. Regression of Hodgkin’s disease after measles. Lancet (Lond., Engl.) 1981;1:1112. - PubMed
- Dock G. The influence of complicating diseases upon leukaemia. Am. J. Med. Sci. 1904;127:563.
- Ono S, Hattori O, Nagai Y, Nagata I. Oncolytic effect of influenza virus upon Ehrlich carcinoma and Yoshida ascites hepatoma. Gan. 1955;46:512–514. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials