Andrographis paniculata and Its Bioactive Diterpenoids Against Inflammation and Oxidative Stress in Keratinocytes - PubMed (original) (raw)
Andrographis paniculata and Its Bioactive Diterpenoids Against Inflammation and Oxidative Stress in Keratinocytes
Eugenie Mussard et al. Antioxidants (Basel). 2020.
Abstract
Andrographis paniculata was widely used in traditional herbal medicine to treat various diseases. This study explored the potential anti-aging activity of Andrographis paniculata in cutaneous cells. Human, adult, low calcium, high temperature (HaCaT) cells were treated with methanolic extract (ME), andrographolide (ANDRO), neoandrographolide (NEO), 14-deoxyandrographolide (14DAP) and 14-deoxy-11,12-didehydroandrographolide (14DAP11-12). Oxidative stress and inflammation were induced by hydrogen peroxide and lipopolysaccharide/TNF-α, respectively. Reactive oxygen species (ROS) production was measured by fluorescence using a 2',7'-dichlorofluorescein diacetate (DCFH-DA) probe and cytokines were quantified by ELISA for interleukin-8 (IL-8) or reverse transcription-quantitative polymerase chain reaction (RT-qPCR) for tumor necrosis factor-α (TNF-α). Hyaluronic acid (HA) secretion was determined by an ELISA. Our results show a decrease in ROS production and TNF-α expression by ME (5 µg/mL) in HaCaT under pro-oxidant and pro-inflammatory conditions, respectively. ME protected HaCaT against oxidative stress and inflammation. Our findings confirm that ME can be used for the development of bioactive compounds against epidermal damage.
Keywords: Andrographis paniculata; andrographolide; inflammation; keratinocytes; oxidative stress; skin aging.
Conflict of interest statement
To the best of our knowledge, no conflict of interest, financial or other, exists.
Figures
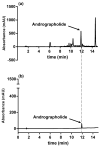
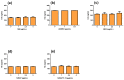
Figure 1
HPLC results of ANDRO from ME of Andrographis paniculata (a); ANDRO standard (b). Abbreviations: ANDRO, andrographolide; ME, methanolic extract.
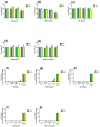
Figure 2
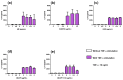
Effect of ME, ANDRO, NEO, 14DAP, and 14DAP11-12 on HaCaT cytotoxicity. HaCaT was treated with increasing concentrations (1, 2.5, or 5 µg/mL) of ME (a,f), ANDRO (b,g), NEO (c,h), 14DAP (d,i), or 14DAP11-12 (e,j) for 24 h and 48 h. The control cells were treated with 0.05% DMSO (noted as “0”). Cell viability was analyzed by mitochondrial metabolism using an MTT assay (a–e). Then, cell cytotoxicity was determined by a dosage of extracellular LDH activity (f–j). Cells untreated with stimuli were a negative control and cells treated with lysis agent were a positive control (noted as “Control”). The values are mean ± SD, ** p < 0.01 compared with control group, n = 3. Abbreviations: NEO, neoandrographolide; 14DAP, 14-deoxyandrographolide; 14DAP11-12, 14-deoxy-11,12-didehydroandrographolide; DMSO, dimethyl sulfoxide; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; LDH, lactate dehydrogenase.
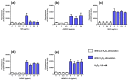
Figure 3
ROS production and the effect of ME, ANDRO, NEO, 14DAP, and 14DAP11-12 in HaCaT. ME (a), ANDRO (b), NEO (c), 14DAP (d), or 14DAP11-12 (e) was used at 1, 2.5, or 5 µg/mL for 1 h. The control cells were treated with 0.05% DMSO (noted as “0”). ROS production was induced by 0.5 mM H2O2 and free radical scavenging activity was done using a DCFH-DA probe. The values are mean ± SD, * p < 0.05 compared with control group, n = 3. Abbreviations: ROS, reactive oxygen species; DCFH-DA, 2′,7′-dichlorofluorescein diacetate.
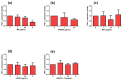
Figure 4
TNF-α expression and effect of ME, ANDRO, NEO, 14DAP, and 14DAP11-12 in HaCaT under pro-inflammation conditions. ME (a), ANDRO (b), NEO (c), 14DAP (d), or 14DAP11-12 (e) was used at 1, 2.5, or 5 µg/mL for 24 h. The control cells were treated with 0.05% DMSO (noted as “0”). Inflammation conditions were induced by LPS (10 µg/mL) for 6 h and TNF-α expression was determined by RT-qPCR. The values are mean ± SD, * p < 0.05 compared with control group, n = 3. Abbreviations: LPS, lipopolysaccharides; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; TNF-α, tumor necrosis factor-α.
Figure 5
IL-8 secretion and effect of ME, ANDRO, NEO, 14DAP, and 14DAP11-12 in HaCaT under pro-inflammation conditions. ME (a), ANDRO (b), NEO (c), 14DAP (d), or 14DAP11-12 (e) was used at 1, 2.5, or 5 µg/mL for 24 h. The control cells were treated with 0.05% DMSO (noted as “0”). Inflammation was induced by TNF-α (10 ng/mL) and IL-8 secretion was performed using an ELISA assay. The values are mean ± SD, compared with control group, n = 3. Abbreviations: IL-8, interleukin-8.
Figure 6
HA production and effect of ME, ANDRO, NEO, 14DAP, and 14DAP11-12 in HaCaT. ME (a), ANDRO (b), NEO (c) 14DAP (d), or 14DAP11-12 (e) was used at 1, 2.5 or 5 µg/mL for 48h. HA was determined by an ELISA. The values are mean ± SD, compared with control group, n = 3. Abbreviations: HA, hyaluronic acid.
Similar articles
- Andrographis Paniculata and Its Bioactive Diterpenoids Protect Dermal Fibroblasts Against Inflammation and Oxidative Stress.
Mussard E, Jousselin S, Cesaro A, Legrain B, Lespessailles E, Esteve E, Berteina-Raboin S, Toumi H. Mussard E, et al. Antioxidants (Basel). 2020 May 15;9(5):432. doi: 10.3390/antiox9050432. Antioxidants (Basel). 2020. PMID: 32429312 Free PMC article. - Andrographis paniculata extracts and major constituent diterpenoids inhibit growth of intrahepatic cholangiocarcinoma cells by inducing cell cycle arrest and apoptosis.
Suriyo T, Pholphana N, Rangkadilok N, Thiantanawat A, Watcharasit P, Satayavivad J. Suriyo T, et al. Planta Med. 2014 May;80(7):533-43. doi: 10.1055/s-0034-1368399. Epub 2014 Apr 29. Planta Med. 2014. PMID: 24782229 - Andrographis paniculata diterpenoids and ethanolic extract inhibit TNFα-induced ICAM-1 expression in EA.hy926 cells.
Lin HC, Li CC, Yang YC, Chiu TH, Liu KL, Lii CK, Chen HW. Lin HC, et al. Phytomedicine. 2019 Jan;52:157-167. doi: 10.1016/j.phymed.2018.09.205. Epub 2018 Sep 21. Phytomedicine. 2019. PMID: 30599895 - Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancer.
Lim JC, Chan TK, Ng DS, Sagineedu SR, Stanslas J, Wong WS. Lim JC, et al. Clin Exp Pharmacol Physiol. 2012 Mar;39(3):300-10. doi: 10.1111/j.1440-1681.2011.05633.x. Clin Exp Pharmacol Physiol. 2012. PMID: 22017767 Review. - Review on liver inflammation and antiinflammatory activity of Andrographis paniculata for hepatoprotection.
Chua LS. Chua LS. Phytother Res. 2014 Nov;28(11):1589-98. doi: 10.1002/ptr.5193. Epub 2014 Jul 10. Phytother Res. 2014. PMID: 25043965 Review.
Cited by
- The Modulatory Influence of Plant-Derived Compounds on Human Keratinocyte Function.
Merecz-Sadowska A, Sitarek P, Zajdel K, Kucharska E, Kowalczyk T, Zajdel R. Merecz-Sadowska A, et al. Int J Mol Sci. 2021 Nov 19;22(22):12488. doi: 10.3390/ijms222212488. Int J Mol Sci. 2021. PMID: 34830374 Free PMC article. Review. - Ethnobotanical Survey on Bitter Tea in Taiwan.
Chao J, Chen TY, Pao LH, Deng JS, Cheng YC, Su SY, Huang SS. Chao J, et al. Front Pharmacol. 2022 Feb 18;13:816029. doi: 10.3389/fphar.2022.816029. eCollection 2022. Front Pharmacol. 2022. PMID: 35250565 Free PMC article. - Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials.
Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Nur H, Ismail AF, Sharif S, RamaKrishna S, Berto F. Parham S, et al. Antioxidants (Basel). 2020 Dec 21;9(12):1309. doi: 10.3390/antiox9121309. Antioxidants (Basel). 2020. PMID: 33371338 Free PMC article. Review. - Review: Effect of Experimental Diets on the Microbiome of Productive Animals.
Huaiquipán R, Quiñones J, Díaz R, Velásquez C, Sepúlveda G, Velázquez L, Paz EA, Tapia D, Cancino D, Sepúlveda N. Huaiquipán R, et al. Microorganisms. 2023 Aug 31;11(9):2219. doi: 10.3390/microorganisms11092219. Microorganisms. 2023. PMID: 37764062 Free PMC article. Review. - Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy.
Hossain S, Urbi Z, Karuniawati H, Mohiuddin RB, Moh Qrimida A, Allzrag AMM, Ming LC, Pagano E, Capasso R. Hossain S, et al. Life (Basel). 2021 Apr 16;11(4):348. doi: 10.3390/life11040348. Life (Basel). 2021. PMID: 33923529 Free PMC article. Review.
References
- Pillai S., Oresajo C., Hayward J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources