COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics - PubMed (original) (raw)
Review
. 2020 Oct;50(10):e13338.
doi: 10.1111/eci.13338. Epub 2020 Aug 26.
Affiliations
- PMID: 32589264
- PMCID: PMC7361203
- DOI: 10.1111/eci.13338
Review
COVID-19 and non-alcoholic fatty liver disease: Two intersecting pandemics
Piero Portincasa et al. Eur J Clin Invest. 2020 Oct.
Abstract
Background: Initial evidence from China suggests that most vulnerable subjects to COVID-19 infection suffer from pre-existing illness, including metabolic abnormalities. The pandemic characteristics and high-lethality rate of COVID-19 infection have raised concerns about interactions between virus pathobiology and components of the metabolic syndrome.
Methods: We harmonized the information from the recent existing literature on COVID-19 acute pandemic and mechanisms of damage in non-alcoholic fatty liver disease (NAFLD), as an example of chronic (non-communicable) metabolic pandemic.
Results: COVID-19-infected patients are more fragile with underlying metabolic illness, including hypertension, cardiovascular disease, type 2 diabetes, chronic lung diseases (e.g. asthma, chronic obstructive pulmonary disease and emphysema) and metabolic syndrome. During metabolic abnormalities, expansion of metabolically active fat ('overfat condition') parallels chronic inflammatory changes, development of insulin resistance and accumulation of fat in configuring NAFLD. The deleterious interplay of inflammatory pathways chronically active in NAFLD and acutely in COVID-19-infected patients, can explain liver damage in a subgroup of patients and might condition a worse outcome in metabolically compromised NAFLD patients. In a subgroup of patients with NAFLD, the underlying liver fibrosis might represent an additional and independent risk factor for severe COVID-19 illness, irrespective of metabolic comorbidities.
Conclusions: NAFLD can play a role in the outcome of COVID-19 illness due to frequent association with comorbidities. Initial evidences suggest that increased liver fibrosis in NAFLD might affect COVID-19 outcome. In addition, long-term monitoring of post-COVID-19 NAFLD patients is advisable, to document further deterioration of liver damage. Further studies are required in this field.
Keywords: SARS-CoV-2; fatty liver; mitochondria; nitrosative stress; oxidative stress.
© 2020 Stichting European Society for Clinical Investigation Journal Foundation. Published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures
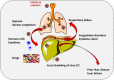
FIGURE 1
Sequences of pathophysiological mechanisms predisposing to metabolic illness and liver steatosis. Rationale to explain multi‐organ and liver damage during COVID‐19 infection. (A) Initial role of wrong lifestyles (hypercaloric, unbalanced, fructose‐ and refined carbohydrate‐enriched diet, sedentary behaviour), on a genetic/racial, ethnical and environmental background. Changes in intestinal microbiota can also govern additional metabolic changes due to biotransformation of foods, local inflammatory changes, increased intestinal permeability to bacterial products (ie lypopolisaccharides). (B) Expansion of visceral fat may occur in different phenotypes, independently of simple body weight (encompassing the term 'adiposity' or 'overfat'). The three subtypes at risk include normal weight but metabolically obese subjects (characterized by high visceral adiposity, ie about % overfat, normal lean mass, propensity to develop metabolic abnormalities),, overweight individuals and obese sarcopaenic subjects (high visceral adiposity, decreased lean mass, likely several metabolic abnormalities). The subtype «normal weight obese» has increased (>30%) fat mass (not necessarily visceral adiposity), a normal lean mass, without metabolic abnormalities. Overfat conditions (in red) are predisposing to chronic metabolic inflammation, compromised immunity, increased risk of chronic disease and infections (including viral infections). Underweight, underfat individuals also share the same risk for chronic inflammation, compromised immunity, increased risk of chronic disease and infections. (C) The metabolically active vicious circle originates from the excess visceral fat with production of inflammatory molecules. In lean individuals or metabolically healthy subjects, anti‐inflammatory cytokines (transforming growth factor beta (TGF‐β), interleukin 10 (IL‐10), IL‐4, IL‐13, nitric oxide (NO)) activate M2 macrophage‐ and inhibit neutrophil‐mediated inflammation. T lymphocytes, neutrophils, B1 and B2 cells, NK cells and innate lymphoid cells also populate the fat tissue. Hypertrophic or apoptotic adipocytes (in grey) in obese individuals can secrete pro‐inflammatory molecules (leptin, resistin, IL‐6 and tumour necrosis factor‐α) that activate a pro‐inflammatory M1 macrophage. The pro‐inflammatory metabolic status is a factor promoting insulin resistance, as well as defective immune response (poor T cell and macrophage function). (D) Further progression of the chronic pro‐inflammatory status and insulin resistance paves the way to several metabolic risk factors contributing to the metabolic syndrome. (E) Chronic illness can follow with established risk factors. (F) Non‐alcoholic fatty liver disease (NAFLD) and the spectrum of liver abnormalities are the consequence of the accumulated metabolic abnormalities. Excess lipolysis during insulin resistance will increase the influx of free fatty acids (FFA), synthesis of triglycerides, enrichment of FFA pool with lipotoxic products (lysophosphatidylcholine (LPC); diacylglycerol (DAG); ceramides). Products mediate endoplasmic reticulum (ER) stress, oxidant stress and activation of the inflammasome (multiprotein cytoplasmic complex that responds to damage‐associated molecular patterns (DAMPs), as part of the innate immunity response)., , , Abbreviations: BMI, body mass index
FIGURE 2
Major factors involved in COVID‐19 infection and liver damage. Factors include lung involvement leading to hypoxia and venous congestion with liver stasis, role of immune cells and cytokines, drug‐induced liver damage and addition of coagulation disorders and cytokine storm. A prior liver disease might exaggerate the damage from ongoing COVID‐19 infection. Non‐alcoholic fatty liver disease might represent per se a condition of intrinsic frailty (due to ongoing lipotoxicity, chronic inflammatory status, insulin resistance, oxidant stress, immune response), or be a marker of additional coexisting metabolic disorders which will aggravate the clinical course of COVID‐19
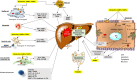
FIGURE 3
Population of innate immune cells playing a role in progression of NAFLD. Immune cells include mast cells (MC), Kupffer cells (KC), neutrophils, dendritic cells (DC), liver sinusoidal endothelial cells (LSEC), resident innate‐like lymphocytes (ILC) and hepatocytes. Kupffer cells, neutrophils, dendritic cells, liver sinusoidal endothelial cells and hepatocytes detect the presence of gut‐derived P/MAMPs (microbe‐associated molecular pattern molecules), endogenous DAMPs (damage‐associated molecular pattern Molecules), PAMPs (pathogen‐associated molecular pattern molecules) and excessive metabolites via PRRs (pattern recognition receptor), leading to the increased release of pro‐inflammatory cytokines and chemokines. In liver sinusoidal endothelial cells, stressor‐induced upregulation of expression of adhesion molecules plus chemokines, stimulate recruitment of neutrophils and monocytes to the liver. Activated neutrophils initiate liver damage mainly by releasing enzymes and ROS (reactive oxygen species). Activated dendritic cells also present antigens to T cells with initiation of adaptive responses. Kupffer cells and hepatocytes regulate release and endocytosis of APPs (acute‐phase protein), thus extending their innate immune function to extrahepatic organs. Kupffer cells, mast cells and hepatocytes increase expression of other factors MMPs (matrix metalloprotease), Ang II (angiotensin II), TGF (transforming growth factor) and HGF (hepatic growth factor) to stimulate HSC (hepatic stellate cell) activation and liver fibrosis. Innate immune signals also mediate metabolic changes (e.g. lipogenesis and insulin resistance) and cell apoptosis, pyroptosis or necrosis in hepatocytes. KCs and LESCs express high levels of SR (scavenger receptor), which clears circulating molecules and organisms. SR plays a key role in the innate immune response. Innate‐like lymphocytes, including NKs (natural killer cell), ILCs (innate lymphoid cell), iNKTs (invariant natural killer T cell) and MAITs (mucosal‐associated invariant T cell), also generate multiple cytokines and influence their local microenvironment of the liver. ILC are fundamental cell that transit from an immune‐tolerant state (a condition in which they produce interleukin (IL‐10), transforming growth factor (TGF‐β), etc) to an immune‐active phenotype (producing IL‐1s, TNF‐α, etc). ILC form the first line of defence against invading organisms and environmental challenges through pattern recognition receptor (PRR) ligation and activation of complement receptors (CRs) or scavenger receptors (SRs). Together, these events result in liver steatosis, inflammation and fibrosis and lead to NASH and advanced complications. Abbreviations: CCL2, C‐C motif chemokine 2; TF, transcriptional factor; Th1, T helper 1 (Adapted from Cai et al, and Jenne & Kubes117)
Similar articles
- In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19.
Forlano R, Mullish BH, Mukherjee SK, Nathwani R, Harlow C, Crook P, Judge R, Soubieres A, Middleton P, Daunt A, Perez-Guzman P, Selvapatt N, Lemoine M, Dhar A, Thursz MR, Nayagam S, Manousou P. Forlano R, et al. PLoS One. 2020 Oct 8;15(10):e0240400. doi: 10.1371/journal.pone.0240400. eCollection 2020. PLoS One. 2020. PMID: 33031439 Free PMC article. - MAFLD in COVID-19 patients: an insidious enemy.
Dongiovanni P, Meroni M, Longo M, Fracanzani AL. Dongiovanni P, et al. Expert Rev Gastroenterol Hepatol. 2020 Oct;14(10):867-872. doi: 10.1080/17474124.2020.1801417. Epub 2020 Aug 4. Expert Rev Gastroenterol Hepatol. 2020. PMID: 32705906 - Non-Alcoholic Fatty Liver Disease and COVID-19-Two Pandemics Hitting at the Same Time.
Vranić L, Radovan A, Poropat G, Mikolašević I, Milić S. Vranić L, et al. Medicina (Kaunas). 2021 Oct 3;57(10):1057. doi: 10.3390/medicina57101057. Medicina (Kaunas). 2021. PMID: 34684094 Free PMC article. Review. - Aging, Male Sex, Obesity, and Metabolic Inflammation Create the Perfect Storm for COVID-19.
Mauvais-Jarvis F. Mauvais-Jarvis F. Diabetes. 2020 Sep;69(9):1857-1863. doi: 10.2337/dbi19-0023. Epub 2020 Jul 15. Diabetes. 2020. PMID: 32669390 Free PMC article. - Histopathology and genetic susceptibility in COVID-19 pneumonia.
von der Thüsen J, van der Eerden M. von der Thüsen J, et al. Eur J Clin Invest. 2020 Jul;50(7):e13259. doi: 10.1111/eci.13259. Epub 2020 Jun 27. Eur J Clin Invest. 2020. PMID: 32353898 Free PMC article. Review.
Cited by
- Changes in mortality due to Chronic Liver Diseases (CLD) during the COVID-19 pandemic: Data from the United States' National Vital Statistics System.
Paik JM, Shah D, Eberly K, Golabi P, Henry L, Younossi ZM. Paik JM, et al. PLoS One. 2024 Sep 3;19(9):e0289202. doi: 10.1371/journal.pone.0289202. eCollection 2024. PLoS One. 2024. PMID: 39226267 Free PMC article. - Impact of non-alcoholic fatty liver disease on coronavirus disease 2019: A systematic review.
Moeed A, Larik MO, Fahim MAA, Rahman HAU, Najmi L, Changez MIK, Javed MM, Hasibuzzaman MA. Moeed A, et al. World J Hepatol. 2024 Aug 27;16(8):1185-1198. doi: 10.4254/wjh.v16.i8.1185. World J Hepatol. 2024. PMID: 39221098 Free PMC article. - Causal associations between severe COVID-19 and diseases of seven organs: a proteome-wide mendelian randomization study.
Shen Y, Zhang Y, Xu YY, Li X, Wu J, Pei H, Wang L, Zhu T. Shen Y, et al. Front Genet. 2024 Aug 13;15:1421824. doi: 10.3389/fgene.2024.1421824. eCollection 2024. Front Genet. 2024. PMID: 39192889 Free PMC article. - Recent advances in COVID-19-induced liver injury: causes, diagnosis, and management.
Antar SA, Ashour NA, Hamouda AO, Noreddin AM, Al-Karmalawy AA. Antar SA, et al. Inflammopharmacology. 2024 Oct;32(5):2649-2680. doi: 10.1007/s10787-024-01535-7. Epub 2024 Aug 10. Inflammopharmacology. 2024. PMID: 39126569 Review. - A novel peptide encoded by circ-SLC9A6 promotes lipid dyshomeostasis through the regulation of H4K16ac-mediated CD36 transcription in NAFLD.
Wang Y, Tian X, Wang Z, Liu D, Zhao X, Sun X, Tu Z, Li Z, Zhao Y, Zheng S, Yao J. Wang Y, et al. Clin Transl Med. 2024 Aug;14(8):e1801. doi: 10.1002/ctm2.1801. Clin Transl Med. 2024. PMID: 39107881 Free PMC article.
References
- World Health Organization . Coronavirus disease 2019 (COVID 19). Situation Report ‐ 72. Geneva: World Health Organization; 2020.
- European Centre for Disease Prevention and Control . COVID‐19 pandemic, 2020. https://www.ecdc.europa.eu/en/covid‐19‐pandemic. Accessed July 2, 2020.
- Albano D, Bertagna F, Bertoli M, et al. Incidental findings suggestive of Covid‐19 in asymptomatic patients undergoing nuclear medicine procedures in a high prevalence region. J Nucl Med. 2020;61(5):632‐636. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous