Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse - PubMed (original) (raw)
Review
. 2021 Apr 20;34(12):936-961.
doi: 10.1089/ars.2020.8153. Epub 2020 Jul 23.
Affiliations
- PMID: 32597195
- PMCID: PMC8035927
- DOI: 10.1089/ars.2020.8153
Review
Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse
Richard T Premont et al. Antioxid Redox Signal. 2021.
Abstract
Significance: Red blood cell (RBC)-mediated vasodilation plays an important role in oxygen delivery. This occurs through hemoglobin actions, at least in significant part, to convert heme-bound nitric oxide (NO) (in tense [T]/deoxygenated-state hemoglobin) into vasodilator S-nitrosothiol (SNO) (in relaxed [R]/oxygenated-state hemoglobin), convey SNO through the bloodstream, and release it into tissues to increase blood flow. The coupling of hemoglobin R/T state allostery, both to NO conversion into SNO and to SNO release (along with oxygen), under hypoxia supports the model of a three-gas respiratory cycle (O2/NO/CO2). Recent Advances: Oxygenation of tissues is dependent on a single, strictly conserved Cys residue in hemoglobin (βCys93). Hemoglobin couples SNO formation/release at βCys93 to O2 binding/release at hemes ("thermodynamic linkage"). Mice bearing βCys93Ala hemoglobin that is unable to generate SNO-βCys93 establish that SNO-hemoglobin is important for R/T allostery-regulated vasodilation by RBCs that couple blood flow to tissue oxygenation. Critical Issues: The model for RBC-mediated vasodilation originally proposed by Stamler et al. in 1996 has been largely validated: SNO-βCys93 forms in vivo, dilates blood vessels, and is hypoxia-regulated, and RBCs actuate vasodilation proportionate to hypoxia. Numerous compensations in βCys93Ala animals to alleviate tissue hypoxia (discussed herein) are predicted to preserve vasodilatory responses of RBCs but impair linkage to R/T transition in hemoglobin. This is borne out by loss of responsivity of mutant RBCs to oxygen, impaired blood flow responses to hypoxia, and tissue ischemia in βCys93-mutant animals. Future Directions: SNO-hemoglobin mediates hypoxic vasodilation in the respiratory cycle. This fundamental physiology promises new insights in vascular diseases and blood disorders.
Keywords: S-nitrosohemoglobin; S-nitrosothiol; S-nitrosylation; autoregulation; hypoxic vasodilation.
Conflict of interest statement
Drs. Stamler and Reynolds hold patents related to renitrosylation of hemoglobin and erythrocytes. Case Western Reserve University and University Hospitals have appropriate conflict management plans in place.
Figures
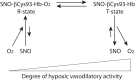
FIG. 1.
Thermodynamic linkage of βCys93 reactivity to Hb oxygenation state and conformation. Hb βCys93 is highly reactive when Hb is oxygenated and in the R state, and consequently, SNO at this site forms when oxygen binds, whereas βCys93 is unreactive (and SNO is disfavored) when Hb is deoxygenated and in the T state (13, 14, 63, 66). Thus, Hb can carry SNO in the R state and release it along with oxygen upon transition to the T state in hypoxic tissue in a graded manner. Hb, hemoglobin; SNO, S-nitrosothiol.
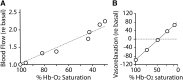
FIG. 2.
Autoregulation of tissue blood flow under hypoxia. (A) Blood flow through the canine hind limb increases linearly as Hb O2-saturation decreases. Data replotted from Ross et al. (100). (B) Allosteric modulation of vasodilation. Vasodilatory activity of RBCs varies with oxygenation state and effector thiol. Organ bath assay of phenylephrine-preconstricted aorta for vasorelaxation activity of human RBCs was performed in the presence of 10 μ_M_ glutathione at various O2 levels. Oxygenated RBCs/SNO-Hb is vasoconstrictive, due to scavenging of endogenous NO, but increasing vasorelaxation occurs as O2 level is dropped, reflecting SNO release. Data replotted from McMahon et al. (66) with permission. NO, nitric oxide; RBCs, red blood cells; SNO-Hb, S-nitrosylated hemoglobin.
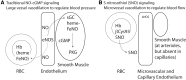
FIG. 3.
Mechanisms for NO bioactivity. (A) Traditional signaling by NO gas. Vasodilatory substances in blood activate eNOS to generate NO gas. Gaseous diffusion into adjacent vascular smooth muscle cells allows NO to bind to heme in sGC to generate cGMP, which activates cGMP-activated PKG to promote muscle relaxation and vasodilation. NO gas diffusion into the bloodstream leads to NO binding to heme in Hb, where it is unable to release in the free form; this removal of free NO is thus vasoconstrictive. (B) Signaling by SNO. NO gas can be redox-activated to form nitrosonium that can react with free cysteine residues to create protein SNO. Hb can utilize the Fe in heme to generate SNO at βCys93, that is, Hb acts as an SNO synthase using NO, SNO, or nitrite. Released SNO is vasodilatory at endothelial cells and arteriole smooth muscle cells. cGMP, cyclic GMP; eNOS, endothelial nitric oxide synthase; PKG, protein kinase G; sGC, soluble guanylyl cyclase.
FIG. 4.
Schematic of humanized mouse model. The C93 mouse carries normal human adult Hb (HbA) in its mouse RBCs, because it has the human α-globin and β-globin genes in place of their mouse counterparts. The C93A mouse additionally has a point mutation of the human β-globin gene to replace Cys93 with Ala that is unable to carry SNO. Fetal Hb C93 is always present.
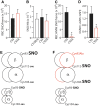
FIG. 5.
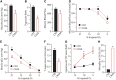
SNO in humanized C93A RBCs. (A) RBC total SNO content is unaltered by C93A mutation. (B) Isolated Hb total SNO content is unaltered by C93A mutation. (C) SNO content of C93A RBC lysate, separated into a >10 kDa fraction (containing Hb but not low-molecular-weight thiols), is significantly reduced (43) but includes new SNO in α- and β-globin chains (134). (D) GSNO content of C93A RBCs is significantly increased. (E, F) Schematic diagrams of SNO carried by HbA (α2β2) and smaller Hb populations of HbF (α2γ2) and HbD (α2δ2) in C93 mice (E), versus compensatory changes in SNO-Hbs in C93A mice (F), based on data in (134). *p < 0.05 for C93 versus C93A. (A, C) Replotted from Isbell et al. (43) with permission, (B, D) replotted from Zhang et al. (134) with permission. GSNO, SNO-glutathione.
FIG. 6.
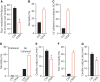
Active and reactive hyperemia are reduced in C93A mice. (A) Oxygenation of the gastrocnemius muscle is significantly lower in C93A mice at baseline. (B) Decreased liter size in C93A pairs compared with C93 pairs, suggesting poor survival during development. (C) Basal blood flow through the gastrocnemius muscle (in Doppler units) is significantly lower in C93A mice. (D) Blood flow through the gastrocnemius muscle is significantly lower in C93A mice as the oxygen content of inspired air (FiO2) is reduced from the normal 21% to 5%. (E) Oxygenation of the gastrocnemius muscle is significantly lower in C93A mice as the oxygen content of inspired air (FiO2) is reduced from the normal 21% to 5%. (F) Electrocardiogram T-wave area, which is correlated with cardiac tissue oxygenation, is significantly decreased in hypoxic C93A mice breathing room air, evidence of basal heart muscle oxygen deficiency. (G) Electrocardiogram hyperacute T, ST-wave area is significantly increased in hypoxic C93A mice breathing air with reduced oxygen content, reflective of ischemia and injury. (H) Mortality is significantly increased in C93A mice after 5 min exposure to 5% oxygen environment. *p < 0.05 for C93 versus C93A. Replotted from Zhang et al. (134) with permission. FiO2, fraction of inspired oxygen.
FIG. 7.
Impaired blood flow as cause of cardiac injury and heart failure in C93A mice. (A) Reactive hyperemia is significantly blunted in C93A mice, measured as elevated blood flow over basal after release of 5 min blockade of blood flow to the gastrocnemius muscle. (B) Increased mortality in C93A mice following cardiac ischemia–reperfusion. (C) Increased presence of collateral vessels from the left or right coronary artery supplying the left ventricle in C93A mice. (D) Mortality following cardiac ischemia–reperfusion occurs only in mice lacking collateral vessels. (E) Reduced cardiac output in C93A mice after acute (2 day) TAC pressure overload-induced heart failure. (F) Increased mortality in C93A mice following chronic (28 day) TAC. (G) Reduced cardiac output in C93A mice after chronic TAC. *p < 0.05 for C93 versus C93A. Replotted from Zhang et al. (135) with permission. TAC, transverse aortic constriction.
FIG. 8.
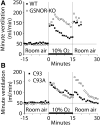
SNO-mediated regulation of ventilatory physiology. (A) Male mice lacking GSNOR (Adh5), an enzyme that reduces tissue SNO levels, lack respiratory roll-off during exposure to hypoxia (10% oxygen) since their minute ventilation fails to return to baseline like that of WT control mice. (B) C93A mice have augmented short-term potentiation of ventilation (measured as minute ventilation) following return to room air (21% oxygen) after brief hypoxia (10% oxygen). Replotted from Palmer et al. (77) (A) and Gaston et al. (26) (B), with permissions. GSNOR, glutathione-SNO reductase; WT, wild type.
Similar articles
- Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via _β_Cys93.
Hausladen A, Qian Z, Zhang R, Premont RT, Stamler JS. Hausladen A, et al. J Pharmacol Exp Ther. 2022 Jul;382(1):1-10. doi: 10.1124/jpet.122.001194. Epub 2022 May 5. J Pharmacol Exp Ther. 2022. PMID: 35512801 Free PMC article. - Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia.
Zhang R, Hess DT, Qian Z, Hausladen A, Fonseca F, Chaube R, Reynolds JD, Stamler JS. Zhang R, et al. Proc Natl Acad Sci U S A. 2015 May 19;112(20):6425-30. doi: 10.1073/pnas.1502285112. Epub 2015 Mar 25. Proc Natl Acad Sci U S A. 2015. PMID: 25810253 Free PMC article. - Essential Role of Hemoglobin βCys93 in Cardiovascular Physiology.
Premont RT, Stamler JS. Premont RT, et al. Physiology (Bethesda). 2020 Jul 1;35(4):234-243. doi: 10.1152/physiol.00040.2019. Physiology (Bethesda). 2020. PMID: 32490751 Free PMC article. Review. - SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation.
Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. Isbell TS, et al. Nat Med. 2008 Jul;14(7):773-7. doi: 10.1038/nm1771. Epub 2008 May 30. Nat Med. 2008. PMID: 18516054 Free PMC article. - Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Circ Res. 2020 Jan 3;126(1):129-158. doi: 10.1161/CIRCRESAHA.119.315626. Epub 2019 Oct 8. Circ Res. 2020. PMID: 31590598 Free PMC article. Review.
Cited by
- The Ever-Expanding Influence of the Endothelial Nitric Oxide Synthase.
Rafea R, Siragusa M, Fleming I. Rafea R, et al. Basic Clin Pharmacol Toxicol. 2025 May;136(5):e70029. doi: 10.1111/bcpt.70029. Basic Clin Pharmacol Toxicol. 2025. PMID: 40150952 Free PMC article. Review. - S-nitroso-L-cysteine stereoselectively blunts the adverse effects of morphine on breathing and arterial blood gas chemistry while promoting analgesia.
Getsy PM, Young AP, Bates JN, Baby SM, Seckler JM, Grossfield A, Hsieh YH, Lewis THJ, Jenkins MW, Gaston B, Lewis SJ. Getsy PM, et al. Biomed Pharmacother. 2022 Sep;153:113436. doi: 10.1016/j.biopha.2022.113436. Epub 2022 Jul 26. Biomed Pharmacother. 2022. PMID: 36076552 Free PMC article. - Emerging concepts in the molecular cell biology and functions of mammalian erythrocytes.
Kumar SD, Ghosh J, Ghosh S, Eswarappa SM. Kumar SD, et al. J Biol Chem. 2025 Apr;301(4):108331. doi: 10.1016/j.jbc.2025.108331. Epub 2025 Feb 19. J Biol Chem. 2025. PMID: 39984047 Free PMC article. Review. - A natural heme deficiency exists in biology that allows nitric oxide to control heme protein functions by regulating cellular heme distribution.
Stuehr DJ, Biswas P, Dai Y, Ghosh A, Islam S, Jayaram DT. Stuehr DJ, et al. Bioessays. 2023 Aug;45(8):e2300055. doi: 10.1002/bies.202300055. Epub 2023 Jun 5. Bioessays. 2023. PMID: 37276366 Free PMC article. - S-Nitrosylated hemoglobin predicts organ yield in neurologically-deceased human donors.
Nazemian R, Matta M, Aldamouk A, Zhu L, Awad M, Pophal M, Palmer NR, Armes T, Hausladen A, Stamler JS, Reynolds JD. Nazemian R, et al. Sci Rep. 2022 Apr 22;12(1):6639. doi: 10.1038/s41598-022-09933-z. Sci Rep. 2022. PMID: 35459243 Free PMC article.
References
- Arthur HM, Campbell P, Harvey PJ, McGillion M, Oh P, Woodburn E, and Hodgson C. Women, cardiac syndrome X, and microvascular heart disease. Can J Cardiol 28: S42–S49, 2012 - PubMed
- Azizi F, Kielbasa JE, Adeyiga AM, Maree RD, Frazier M, Yakubu M, Shields H, King SB, and Kim–Shapiro DB. Rates of nitric oxide dissociation from hemoglobin. Free Radic Biol Med 39: 145–151, 2005 - PubMed
- Basu S, Wang X, Gladwin MT, and Kim-Shapiro DB. Chemiluminescent detection of S-nitrosated proteins: comparison of tri-iodide, copper/CO/cysteine, and modified copper/cysteine methods. Methods Enzymol 440: 137–156, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources