Potential Role of Neuroactive Tryptophan Metabolites in Central Fatigue: Establishment of the Fatigue Circuit - PubMed (original) (raw)
Review
Potential Role of Neuroactive Tryptophan Metabolites in Central Fatigue: Establishment of the Fatigue Circuit
Masatoshi Yamashita. Int J Tryptophan Res. 2020.
Abstract
Central fatigue leads to reduced ability to perform mental tasks, disrupted social life, and impaired brain functions from childhood to old age. Regarding the neurochemical mechanism, neuroactive tryptophan metabolites are thought to play key roles in central fatigue. Previous studies have supported the "tryptophan-serotonin enhancement hypothesis" in which tryptophan uptake into extensive brain regions enhances serotonin production in the rat model of exercise-induced fatigue. However, serotonin was transiently released after 30 minutes of treadmill running to exhaustion, but this did not reflect the duration of fatigue. In addition, as the vast majority of tryptophan is metabolized along the kynurenine pathway, possible involvement of the tryptophan-kynurenine pathway in the mechanism of central fatigue induction has been pointed out. More recently, our study demonstrated that uptake of tryptophan and kynurenine derived from the peripheral circulation into the brain enhances kynurenic acid production in rat brain in sleep deprivation-induced central fatigue, but without change in serotonin activity. In particular, dynamic change in glial-neuronal interactive processes within the hypothalamus-hippocampal circuit causes central fatigue. Furthermore, increased tryptophan-kynurenine pathway activity in this circuit causes reduced memory function. This indicates a major potential role for the endogenous tryptophan-kynurenine pathway in central fatigue, which supports the "tryptophan-kynurenine enhancement hypothesis." Here, we review research on the basic neuronal mechanism underlying central fatigue induced by neuroactive tryptophan metabolites. Notably, these basic findings could contribute to our understanding of latent mental problems associated with central fatigue.
Keywords: Central fatigue; glial-neuronal interactions; neuroactive tryptophan metabolites.
© The Author(s) 2020.
Conflict of interest statement
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Figure 1.
Factors affecting each type of fatigue. Fatigue is mainly divided into 3 types: central fatigue, peripheral fatigue, and infection fatigue. Because of their neurochemical mechanisms, recovery from central fatigue and infection fatigue is more difficult without sufficient rest and supplements (or medicines) relative to peripheral fatigue.
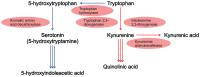
Figure 2.
The neuroactive tryptophan pathway and metabolites. In mammals, as only about 5% of tryptophan is catabolized via the serotonin pathway, the vast majority of tryptophan is metabolized in the kynurenine pathway, which is the precursor pathway for the synthesis of the neuroinhibitory molecule, kynurenic acid, and neurotoxic molecule, quinolinic acid. Is the rate of the kynurenine pathway of tryptophan metabolism involved in central fatigue? If the tryptophan-kynurenine pathway is enhanced during central fatigue, does it lead to a reduction in cognitive functions and severe fatigue?
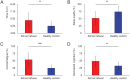
Figure 3.
School refusal children showed higher levels of central fatigue and sleep derangement. (A and B): Compared with healthy control children, school refusal children showed a significant shift in the midpoint of sleep and lower sleep quality. (C and D): Moreover, induction of central fatigue and decreased cognition were significantly greater in school refusal children. The derangement of sleep and induction of central fatigue were higher for the school refusal children. *P < .05, **P < .01.
Figure 4.
Central fatigue becomes more severe as sleep disturbance progresses. (A): In schoolchildren, central fatigue was positively correlated with disturbance of sleep rhythm (r = 0.61, P = .001). (B): In schoolchildren, central fatigue was negatively correlated with sleep efficiency (r = −0.50, P = .01). These results indicate that higher level of central fatigue is influenced by sleep disturbance.
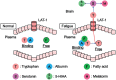
Figure 5.
The uptake of peripheral tryptophan by the brain. Tryptophan binds to albumin in the blood under normal conditions. Non-esterified fatty acids (NEFA) also compete for the same binding site. An increase in the levels of NEFA results in the dissociation of albumin and tryptophan during exercise-induced fatigue and postoperative-induced fatigue. Then, free tryptophan is rapidly taken into the brain via system L transporter located on the surface of the blood-brain barrier, and thus leading to enhanced serotonin synthesis in the brain by the enzymatic activity of tryptophan hydroxylase 2. 5-HIAA indicates 5-hydroxyindoleacetic acid; LAT-1, L-type amino acid transporter 1.
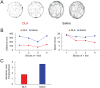
Figure 6.
The effect of tryptophan receptor agonist administration on biobehavioral activities. (A and B): Rats administered DLA had lower spontaneous locomotor activities in the open field than rats administered saline. (C): Serotonin concentration in the hypothalamus decreased in rats injected with DLA compared with rats injected with saline. This result indicates that tryptophan receptor agonist induces the reduction of locomotor activity and serotonin level, although most studies have reported that serotonin activity in the brain is associated with central fatigue. It is possible that tryptophan-serotonin pathway is not involved in central fatigue. DLA indicates
d
,
l
-β-(1-naphthyl)alanine.
Figure 7.
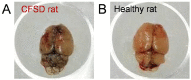
Disruption of the blood-brain barrier (BBB) by sleep deprivation–induced central fatigue. The breakdown of the BBB can be estimated by quantification of extravasated Evans Blue in the brain. Using this method, extravasated Evans Blue content in the whole brain increased in central fatigue induced by (A) chronic sleep disorder (CFSD) rats compared with (B) healthy rats. This result indicates that central fatigue could lead to increased BBB permeability, suggesting barrier breakdown. Perhaps, tryptophan and other substances can freely enter the brain without the assistance of some transporter.
Figure 8.
Metabolism of tryptophan to kynurenine during central fatigue. Tryptophan is catabolized along the kynurenine pathway by tryptophan dioxygenase (TDO) or indoleamine dioxygenase in the liver, and then crosses the blood-brain barrier (BBB) to be rapidly taken up in the brain. Also, tryptophan may be directly metabolized to kynurenine by the enzymatic activity of TDO in the brain. In central fatigue, tryptophan and kynurenine enter the brain via the BBB in a synergetic manner. LAT-1 indicates L-type amino acid transporter 1.
Figure 9.
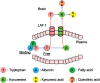
The role of the fatigue circuit: from blood to brain. There are 3 stages of central fatigue induction. Stage 1 is marked by synergetic transfer of tryptophan and kynurenine from blood to brain. Stage 2 is indicated by the rise of brain tryptophan and kynurenine to excessive levels and detection of tryptophan-kynurenic acid pathway activity at the glial-neuronal interactive level within the hypothalamus-hippocampal circuit. Stage 3 is the impairment of cognitive functions. The fatigue circuit includes the tryptophan-kynurenine-kynurenic acid pathway signals generated at neuronal-neuronal and glial-neuronal synapses between the peripheral and central nervous systems. Enhancement of pathway activity triggers cognitive dysfunction. LAT-1 indicates L-type amino acid transporter 1.
Similar articles
- Tryptophan circuit in fatigue: From blood to brain and cognition.
Yamashita M, Yamamoto T. Yamashita M, et al. Brain Res. 2017 Nov 15;1675:116-126. doi: 10.1016/j.brainres.2017.09.002. Epub 2017 Sep 8. Brain Res. 2017. PMID: 28893581 - The relationship between central fatigue and Attention Deficit/Hyperactivity Disorder of the inattentive type.
Yamamoto T. Yamamoto T. Neurochem Res. 2022 Sep;47(9):2890-2898. doi: 10.1007/s11064-022-03693-y. Epub 2022 Aug 11. Neurochem Res. 2022. PMID: 35951201 Free PMC article. - [The importance of the kynurenine pathway in depressive disorders].
Jasionowska J, Filip M, Talarowska M, Gałecki P. Jasionowska J, et al. Pol Merkur Lekarski. 2018 Aug 29;45(266):89-93. Pol Merkur Lekarski. 2018. PMID: 30240376 Review. Polish. - Maternal protein restriction during gestation and lactation in the rat results in increased brain levels of kynurenine and kynurenic acid in their adult offspring.
Honório de Melo Martimiano P, de Sa Braga Oliveira A, Ferchaud-Roucher V, Croyal M, Aguesse A, Grit I, Ouguerram K, Lopes de Souza S, Kaeffer B, Bolaños-Jiménez F. Honório de Melo Martimiano P, et al. J Neurochem. 2017 Jan;140(1):68-81. doi: 10.1111/jnc.13874. Epub 2016 Dec 5. J Neurochem. 2017. PMID: 27778340 - [Kynurenines in pathogenesis of endogenous psychiatric disorders].
Shilov IuE, Bezrukov MV. Shilov IuE, et al. Vestn Ross Akad Med Nauk. 2013;(1):35-41. doi: 10.15690/vramn.v68i1.535. Vestn Ross Akad Med Nauk. 2013. PMID: 23805637 Review. Russian.
Cited by
- Impact of Long-Rope Jumping on Monoamine and Attention in Young Adults.
Yamashita M, Yamamoto T. Yamashita M, et al. Brain Sci. 2021 Oct 13;11(10):1347. doi: 10.3390/brainsci11101347. Brain Sci. 2021. PMID: 34679411 Free PMC article. - Developing a Blood Cell-Based Diagnostic Test for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Using Peripheral Blood Mononuclear Cells.
Xu J, Lodge T, Kingdon C, Strong JWL, Maclennan J, Lacerda E, Kujawski S, Zalewski P, Huang WE, Morten KJ. Xu J, et al. Adv Sci (Weinh). 2023 Oct;10(30):e2302146. doi: 10.1002/advs.202302146. Epub 2023 Aug 31. Adv Sci (Weinh). 2023. PMID: 37653608 Free PMC article. - Thermal treatment enhances the resisting exercise fatigue effect of Phyllanthus emblica L.: novel evidence from tannin conversion in vitro, metabolomics, and gut microbiota community analysis.
Zhang D, Deng X, Li M, Qiu M, Zhang Y, Li G, Jiang Y, Tan P, Fan S, Zheng Y, Lin J, Han L, Huang H. Zhang D, et al. Chin Med. 2023 Oct 1;18(1):127. doi: 10.1186/s13020-023-00835-4. Chin Med. 2023. PMID: 37779204 Free PMC article. - Sex Differences in Tryptophan Metabolism: A Systematic Review Focused on Neuropsychiatric Disorders.
Pais ML, Martins J, Castelo-Branco M, Gonçalves J. Pais ML, et al. Int J Mol Sci. 2023 Mar 22;24(6):6010. doi: 10.3390/ijms24066010. Int J Mol Sci. 2023. PMID: 36983084 Free PMC article. Review. - Induction of distinct neuroinflammatory markers and gut dysbiosis by differential pyridostigmine bromide dosing in a chronic mouse model of GWI showing persistent exercise fatigue and cognitive impairment.
Kozlova EV, Carabelli B, Bishay AE, Liu R, Denys ME, Macbeth JC, Piamthai V, Crawford MS, McCole DF, Zur Nieden NI, Hsiao A, Curras-Collazo MC. Kozlova EV, et al. Life Sci. 2022 Jan 1;288:120153. doi: 10.1016/j.lfs.2021.120153. Epub 2021 Nov 18. Life Sci. 2022. PMID: 34801513 Free PMC article.
References
- Loge JH, Ekeberg O, Kaasa S. Fatigue in the general Norwegian population: normative data and associations. J Psychosom Res. 1998;45:53-65. - PubMed
- Farmer A, Fowler T, Scourfield J, Thapar A. Prevalence of chronic disabling fatigue in children and adolescents. Br J Psychiatry. 2004;184:477-481. - PubMed
- Castell LM, Yamamoto T, Phoenix J, Newsholme EA. The role of tryptophan in fatigue in different conditions of stress. Adv Exp Med Biol. 1999;467:697-704. - PubMed
- Caseras X, Mataix-Cols D, Giampietro V, et al. Probing the working memory system in chronic fatigue syndrome: a functional magnetic resonance imaging study using the n-back task. Psychosom Med. 2006;68:947-955. - PubMed
- Cook DB, O’Connor PJ, Lange G, Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage. 2007;36:108-122. - PubMed
Publication types
LinkOut - more resources
Full Text Sources