Effects of Dietary Carbohydrate Content on Circulating Metabolic Fuel Availability in the Postprandial State - PubMed (original) (raw)
Effects of Dietary Carbohydrate Content on Circulating Metabolic Fuel Availability in the Postprandial State
Kim J Shimy et al. J Endocr Soc. 2020.
Abstract
Context: According to the carbohydrate-insulin model of obesity, an elevated insulin-to-glucagon ratio in response to a high-carbohydrate diet directs metabolic fuels toward storage, resulting in lower circulating energy.
Objective: To determine differences in total circulating energy post-meal related to dietary carbohydrate.
Design: Ancillary study within the Framingham State Food Study.
Setting: University community.
Participants: 29 adults (aged 20 to 65 years) with overweight or obesity (body mass index ≥25 kg/m2).
Intervention: After achieving 10% to 14% weight loss on a run-in diet, participants were randomized to weight-loss-maintenance test diets varying in carbohydrate content (high-carbohydrate, 60% of total energy, n = 11; moderate-carbohydrate, 40%, n = 8; low-carbohydrate, 20%, n = 10) and controlled for protein (20%). During 24-hour metabolic ward admissions between 10 and 15 weeks on the test diets, metabolic fuels and hormones were measured.
Main outcome measure: Energy availability (EA) based on energy content of blood glucose, beta-hydroxybutyrate, and free fatty acids, in the late postprandial period (180 to 300 minutes). Insulin at 30 minutes into the test meal (Meal Insulin-30) was measured as an effect modifier.
Results: Insulin-to-glucagon ratio was 7-fold higher in participants on the high- vs low-carbohydrate diet (2.5 and 0.36, respectively). Late postprandial EA was 0.58 kcal/L lower on the high- vs low-carbohydrate diet (P < 0.0001), primarily related to suppression of free fatty acids. Early postprandial EA (30 to 180 minutes) declined fastest in the high-carbohydrate group, and Meal Insulin-30 modified this diet effect.
Conclusions: During weight-loss maintenance on a high-carbohydrate diet, late postprandial EA is reduced, consistent with the carbohydrate-insulin model.
Keywords: beta-hydroxybutyrate; carbohydrate; fatty acids; glucose; insulin; obesity.
© Endocrine Society 2020.
Figures
Figure 1:
Participant flow diagram.
Figure 2:
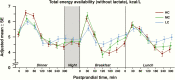
Total energy availability of metabolic fuels (glucose, beta-hydroxybutyrate, free fatty acids) excluding lactate, expressed as adjusted mean ± standard error (SE) in kcal/L, depicted over the 24-hour study period for high carbohydrate (HC), moderate carbohydrate (MC) and low carbohydrate (LC) diets. Postprandial time points (in minutes) and overnight periods are highlighted on the horizontal axis.
Figure 3:
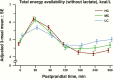
Postprandial total energy availability of metabolic fuels (glucose, beta-hydroxybutyrate, free fatty acids) excluding lactate, expressed as adjusted mean ± standard error (SE) in kcal/L. Postprandial energy availability for all 3 meals (dinner, breakfast, and lunch) were combined into a covariate-adjusted diet-specific mean for high carbohydrate (HC), moderate carbohydrate (MC), and low carbohydrate (LC) at each postprandial time point in minutes.
Figure 4:
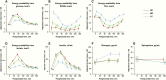
Mean postprandial energy availability of individual fuels are shown in panels A-D (A for glucose, B for beta hydroxybutyrate [BOHB], C for free fatty acids [FFA] and D for lactate). Postprandial energy availability is expressed as the adjusted mean in kcal/L ± standard error (SE) of values at dinner, breakfast, and snack, separated by test diet assignment (high carbohydrate [HC], moderate carbohydrate [MC], low carbohydrate [LC]). Mean concentrations for postprandial insulin, glucagon, and epinephrine are shown in panels E-G (E for insulin in uU/mL, F for glucagon in pg/mL, G for epinephrine in pg/mL). Hormonal concentrations are expressed as the adjusted mean ± standard error (SE) of values at dinner, breakfast, and snack, separated by test diet assignment.
Similar articles
- Effects of diet composition on postprandial energy availability during weight loss maintenance.
Walsh CO, Ebbeling CB, Swain JF, Markowitz RL, Feldman HA, Ludwig DS. Walsh CO, et al. PLoS One. 2013;8(3):e58172. doi: 10.1371/journal.pone.0058172. Epub 2013 Mar 6. PLoS One. 2013. PMID: 23483989 Free PMC article. Clinical Trial. - Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial.
Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak L, Steltz SK, Luoto PK, Wolfe RR, Wong WW, Ludwig DS. Ebbeling CB, et al. BMJ. 2018 Nov 14;363:k4583. doi: 10.1136/bmj.k4583. BMJ. 2018. PMID: 30429127 Free PMC article. Clinical Trial. - Third Exposure to a Reduced Carbohydrate Meal Lowers Evening Postprandial Insulin and GIP Responses and HOMA-IR Estimate of Insulin Resistance.
Lin PJ, Borer KT. Lin PJ, et al. PLoS One. 2016 Oct 31;11(10):e0165378. doi: 10.1371/journal.pone.0165378. eCollection 2016. PLoS One. 2016. PMID: 27798656 Free PMC article. Clinical Trial. - Dietary carbohydrates and insulin action in humans.
Wolever TM. Wolever TM. Br J Nutr. 2000 Mar;83 Suppl 1:S97-102. doi: 10.1017/s0007114500001021. Br J Nutr. 2000. PMID: 10889799 Review. - A Review of Recent Findings on Meal Sequence: An Attractive Dietary Approach to Prevention and Management of Type 2 Diabetes.
Kubota S, Liu Y, Iizuka K, Kuwata H, Seino Y, Yabe D. Kubota S, et al. Nutrients. 2020 Aug 19;12(9):2502. doi: 10.3390/nu12092502. Nutrients. 2020. PMID: 32825124 Free PMC article. Review.
Cited by
- Competing paradigms of obesity pathogenesis: energy balance versus carbohydrate-insulin models.
Ludwig DS, Apovian CM, Aronne LJ, Astrup A, Cantley LC, Ebbeling CB, Heymsfield SB, Johnson JD, King JC, Krauss RM, Taubes G, Volek JS, Westman EC, Willett WC, Yancy WS Jr, Friedman MI. Ludwig DS, et al. Eur J Clin Nutr. 2022 Sep;76(9):1209-1221. doi: 10.1038/s41430-022-01179-2. Epub 2022 Jul 28. Eur J Clin Nutr. 2022. PMID: 35896818 Free PMC article. Review. - Low Carbohydrate Dietary Approaches for People With Type 2 Diabetes-A Narrative Review.
Wheatley SD, Deakin TA, Arjomandkhah NC, Hollinrake PB, Reeves TE. Wheatley SD, et al. Front Nutr. 2021 Jul 15;8:687658. doi: 10.3389/fnut.2021.687658. eCollection 2021. Front Nutr. 2021. PMID: 34336909 Free PMC article. Review. - Alterations in Glucagon Levels and the Glucagon-to-Insulin Ratio in Response to High Dietary Fat or Protein Intake in Healthy Lean Adult Twins: A Post Hoc Analysis.
Schuppelius B, Schüler R, Pivovarova-Ramich O, Hornemann S, Busjahn A, Machann J, Kruse M, Park SQ, Kabisch S, Csanalosi M, Ost AC, Pfeiffer AFH. Schuppelius B, et al. Nutrients. 2024 Nov 15;16(22):3905. doi: 10.3390/nu16223905. Nutrients. 2024. PMID: 39599691 Free PMC article. - Macronutrient Proportions and Fat Type Impact Ketogenicity and Shape the Circulating Lipidome in Dogs.
Jackson MI. Jackson MI. Metabolites. 2022 Jun 24;12(7):591. doi: 10.3390/metabo12070591. Metabolites. 2022. PMID: 35888715 Free PMC article. - The carbohydrate-insulin model: a physiological perspective on the obesity pandemic.
Ludwig DS, Aronne LJ, Astrup A, de Cabo R, Cantley LC, Friedman MI, Heymsfield SB, Johnson JD, King JC, Krauss RM, Lieberman DE, Taubes G, Volek JS, Westman EC, Willett WC, Yancy WS, Ebbeling CB. Ludwig DS, et al. Am J Clin Nutr. 2021 Dec 1;114(6):1873-1885. doi: 10.1093/ajcn/nqab270. Am J Clin Nutr. 2021. PMID: 34515299 Free PMC article.
References
- Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621-628. - PubMed
- Sumithran P, Prendergast LA, Delbridge E, et al. . Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597-1604. - PubMed
- Ludwig DS, Friedman MI. Increasing adiposity: consequence or cause of overeating? JAMA. 2014;311(21):2167-2168. - PubMed
LinkOut - more resources
Full Text Sources