Successful Reduction of Neointimal Hyperplasia on Stainless Steel Coronary Stents by Titania Nanotexturing - PubMed (original) (raw)
Successful Reduction of Neointimal Hyperplasia on Stainless Steel Coronary Stents by Titania Nanotexturing
Aleena Mary Cherian et al. ACS Omega. 2020.
Abstract
Bare metal stents (BMSs) of stainless steel (SS) were surface engineered to develop nanoscale titania topography using a combination of physical vapor deposition and thermochemical processing. The nanoleafy architecture formed on the stent surface remained stable and adherent upon repeated crimping and expansion, as well as under flow. This titania nanoengineered stent showed a preferential proliferation of endothelial cells over smooth muscle cells in vitro, which is an essential requirement for improving the in vivo endothelialization, with concurrent reduction of intimal hyperplasia. The efficacy of this surface-modified stent was assessed after implantation in rabbit iliac arteries for 8 weeks. Significant reduction in neointimal thickening and thereby in-stent restenosis with complete endothelial coverage was observed for the nanotextured stents, compared to BMSs, even without the use of any antiproliferative agents or polymers as in drug-eluting stents. Nanotexturing of stents did not induce any inflammatory response, akin to BMSs. This study thus indicates the effectiveness of a facile titania nanotopography on SS stents for coronary applications and the possibility of bringing this low-priced material back to clinics.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Figure 1
(A) SEM images of the nanotextured SS TNL stent at different magnifications showing uniformity in nanotexturing over the entire surface. (B) AFM images confirming the nanoleafy architecture on SS TNL. (C) EDAX measurements demonstrating the presence of titanium and oxygen on SS TNL.
Figure 2
SEM images of (A) bare SS and (B) SS TNL stents at different magnifications after completion of 20 million cycles under flow. (C) Balloon expansion profiles of bare and nanotextured SS TNL stents.
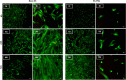
Figure 3
Live–dead fluorescence images of ECs seeded on (A) bare SS and (C) SS TNL stents at 6 [A(i),C(i)], 24 [A(ii),C(ii)], and 72 h [A(iii),C(iii)] and SMCs seeded on (B) bare SS and (D) SS TNL stents at 6 [B(i),D(i)], 24 [B(ii),D(ii)], and 72 h [B(iii),D(iii)].
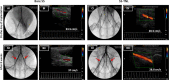
Figure 4
Angiogram and ultrasound images of bare [A(i),B(i)] and SS TNL [C(i),D(i)] stents on the day of implantation and after 8 weeks prior to euthanasia [A(ii),B(ii) for bare; C(ii),D(ii) for SS TNL]. Arrows in the angiogram point to the stented site in the artery. The value for the blood flow velocity of the respective animal is depicted in the ultrasound panel.
Figure 5
H&E images of (A) bare and (B) SS TNL stented vessels at 4× [A(i),B(i)], 10× [A(ii),B(ii)], and 20× [A(iii),B(iii)] magnifications.
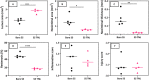
Figure 6
Histomorphometric analysis showing the (A) lumen area (mm2), (B) neointimal area (mm2), (C) neointimal thickness (mm), (D) restenosis (%), (E) inflammation score, and (F) injury score of the bare and nanotextured SS TNL stent-implanted vessel sections.
Figure 7
SEM images of [A(i–iii)] bare SS and [B(i–iii)] nanotextured SS TNL stent-implanted arteries at different magnifications and representative immunofluorescent en face stained images of wheat germ agglutinin on ECs in the [C(i)] bare and [C(ii)] SS TNL stented artery at a depth of 2 μm from the luminal surface (scale bar: 10 μm).
Similar articles
- Coupled benefits of nanotopography and titania surface chemistry in fostering endothelialization and reducing in-stent restenosis in coronary stents.
Cherian AM, Joseph J, Nair MB, Nair SV, Vijayakumar M, Menon D. Cherian AM, et al. Biomater Adv. 2022 Nov;142:213149. doi: 10.1016/j.bioadv.2022.213149. Epub 2022 Oct 12. Biomater Adv. 2022. PMID: 36270158 - Stable Titania Nanostructures on Stainless Steel Coronary Stent Surface for Enhanced Corrosion Resistance and Endothelialization.
Mohan CC, Cherian AM, Kurup S, Joseph J, Nair MB, Vijayakumar M, Nair SV, Menon D. Mohan CC, et al. Adv Healthc Mater. 2017 Jun;6(11). doi: 10.1002/adhm.201601353. Epub 2017 Mar 8. Adv Healthc Mater. 2017. PMID: 28272784 - A novel glycyrrhizin acid-coated stent reduces neointimal formation in a rabbit iliac artery model.
Teng S, Zhu Z, Li Y, Hu X, Fang Z, Liu Z, Zhou S. Teng S, et al. Front Pharmacol. 2023 May 17;14:1159779. doi: 10.3389/fphar.2023.1159779. eCollection 2023. Front Pharmacol. 2023. PMID: 37266147 Free PMC article. - Drug-eluting stents.
García-García HM, Vaina S, Tsuchida K, Serruys PW. García-García HM, et al. Arch Cardiol Mex. 2006 Jul-Sep;76(3):297-319. Arch Cardiol Mex. 2006. PMID: 17091802 Review. - Clinical Effectiveness and Cost Effectiveness of Intracoronary Brachytherapy and Drug Eluting Stents [Internet].
Mørland B, Kløw NE, Rotevatn S, Steigen T, Vatne K, Wisløff T, Kristiansen IS, Norderhaug I. Mørland B, et al. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2004. Report from Norwegian Knowledge Centre for the Health Services (NOKC) No. 08-2004. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2004. Report from Norwegian Knowledge Centre for the Health Services (NOKC) No. 08-2004. PMID: 29320006 Free Books & Documents. Review.
Cited by
- Surface engineering at the nanoscale: A way forward to improve coronary stent efficacy.
Cherian AM, Nair SV, Maniyal V, Menon D. Cherian AM, et al. APL Bioeng. 2021 Jun 1;5(2):021508. doi: 10.1063/5.0037298. eCollection 2021 Jun. APL Bioeng. 2021. PMID: 34104846 Free PMC article. Review. - 3D Printing of Polymeric Bioresorbable Stents: A Strategy to Improve Both Cellular Compatibility and Mechanical Properties.
Sousa AM, Amaro AM, Piedade AP. Sousa AM, et al. Polymers (Basel). 2022 Mar 9;14(6):1099. doi: 10.3390/polym14061099. Polymers (Basel). 2022. PMID: 35335430 Free PMC article. Review. - Micro- and nanoscale biophysical cues for cardiovascular disease therapy.
Mohindra P, Desai TA. Mohindra P, et al. Nanomedicine. 2021 Jun;34:102365. doi: 10.1016/j.nano.2021.102365. Epub 2021 Feb 9. Nanomedicine. 2021. PMID: 33571682 Free PMC article. Review. - What Went Wrong with VEGF-A in Peripheral Arterial Disease? A Systematic Review and Biological Insights on Future Therapeutics.
Kastora SL, Eley J, Gannon M, Melvin R, Munro E, Makris SA. Kastora SL, et al. J Vasc Res. 2022;59(6):381-393. doi: 10.1159/000527079. Epub 2022 Nov 15. J Vasc Res. 2022. PMID: 36380643 Free PMC article. Review. - Mechanical and Electrical Interaction of Biological Membranes with Nanoparticles and Nanostructured Surfaces.
Raval J, Gongadze E, Benčina M, Junkar I, Rawat N, Mesarec L, Kralj-Iglič V, Góźdź W, Iglič A. Raval J, et al. Membranes (Basel). 2021 Jul 14;11(7):533. doi: 10.3390/membranes11070533. Membranes (Basel). 2021. PMID: 34357183 Free PMC article. Review.
References
- van den Heuvel M.; Sorop O.; van Beusekom H. M.; van der Giessen W. J. Endothelial dysfunction after drug eluting stent implantation. Minerva Cardioangiol. 2009, 57, 629–43. - PubMed
LinkOut - more resources
Full Text Sources