Real-world evidence of the effectiveness on glycaemic control of early simultaneous versus later sequential initiation of basal insulin and glucagon-like peptide-1 receptor agonists - PubMed (original) (raw)
. 2020 Dec;22(12):2295-2304.
doi: 10.1111/dom.14154. Epub 2020 Sep 2.
Affiliations
- PMID: 32729183
- PMCID: PMC7818416
- DOI: 10.1111/dom.14154
Real-world evidence of the effectiveness on glycaemic control of early simultaneous versus later sequential initiation of basal insulin and glucagon-like peptide-1 receptor agonists
Julio Rosenstock et al. Diabetes Obes Metab. 2020 Dec.
Abstract
Aim: To assess the impact of the timing of initiating both basal insulin and glucagon-like peptide-1 receptor agonists (GLP-1 RAs) on reaching glycaemic control targets over 6 and 12 months in people with type 2 diabetes (T2D) uncontrolled on oral antihyperglycaemic drugs with an HbA1c of 9% or higher.
Methods: This retrospective cohort study assessed the impact of the timing of initiating both basal insulin and GLP-1 RA therapies on reaching glycaemic targets (HbA1c < 7% and <8%, and ≥1% and ≥2% HbA1c reduction) over 12 months in people with markedly uncontrolled T2D (HbA1c ≥ 9%) on oral antihyperglycaemic drugs identified on the Optum Humedica database (electronic medical records; 1 January 2011 to 30 June 2017). Study cohorts were defined by the days between initiating each injectable: cohort A, 30 days or less (simultaneous initiation) and cohorts B, 31-90, C, 91-180, D, 181-270 and E, 271-360 days (sequential initiation).
Results: Cohort A had the best glycaemic outcomes at 6 and 12 months for all four endpoints, followed by cohort B. The likelihood of achieving an HbA1c of less than 7% did not significantly differ between cohorts A and B (hazard ratio [95% confidence interval]: 0.87 [0.76-1.01]); cohorts C, D and E were significantly less likely to achieve an HbA1c of less than 7% than cohort A (0.62 [0.53-0.72]; 0.62 [0.53-0.72]; 0.63 [0.54-0.73]).
Conclusions: In people with uncontrolled T2D requiring treatment with a GLP-1 RA and basal insulin, greater improvements in glycaemic control were observed when both therapies were initiated within close proximity of one another (≤90 days) compared with initiation 91-360 days apart.
Keywords: GLP-1 analogue; basal insulin; cohort study; database research; glycaemic control; type 2 diabetes.
© 2020 John Wiley & Sons Ltd.
Conflict of interest statement
JR has served on advisory panels for Applied Therapeutics, Boehringer Ingelheim, Eli Lilly, Intarcia, Janssen, Lexicon, Novo Nordisk, Oramed and Sanofi; and has received research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Genentech, GlaxoSmithKline, Intarcia, Janssen, Lexicon, Merck, Novo Nordisk, Oramed, Pfizer and Sanofi. FJAB has served on advisory panels for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, LifeScan, Medtronic, Merck, Novartis, Novo Nordisk, Pfizer, Roche and Sanofi; and has received research support from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, LifeScan Merck, Novo Nordisk, Pfizer, Sanofi and Servier. RL and AB are employees of Sanofi. XVP was employed by Sanofi at the time the study was conducted. LS has received research grants from Chiasma, Genentech and Sanofi; and has stock options with BRAVO4Health. VF has received research grants from Bayer, Boehringer Ingelheim and Gilead; has received honoraria for consulting and lectures from Abbott, Asahi, AstraZeneca, Eli, Intarcia, Novo Nordisk and Takeda; has stock options with BRAVO4Health, Insulin Algorithms and Microbiome Technologies; and has stock in Amgen.
Figures
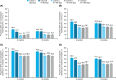
FIGURE 1
Proportion of participants reaching A, HbA1c < 7.0%, B, HbA1c < 8.0%, C, ≥1% reduction in HbA1c from baseline and D, ≥2% reduction in HbA1c from baseline at 6 and 12 months in each cohort. Proportion calculated as number of patients with outcome over the number of patients with ≥1 HbA1c value. CI, confidence interval
FIGURE 2
Characteristics affecting glycaemic control (HbA1c < 7.0%) achievement. Associations calculated by multivariate cox regression model. BI, basal insulin; CCI, Charlson co‐morbidity index; CI, confidence interval; GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; HR, hazard ratio; MI, myocardial infarction; NPH, neutral protamine Hagedorn
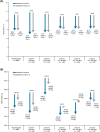
FIGURE 3
Change in A, HbA1c, and B, weight from baseline to months 6 and 12 by cohort. ΔHbA1c is mean change from baseline to month 6 or 12. Δweight is mean change from baseline to month 6 or 12. Baseline weight data include every participant with a baseline weight value. Change from baseline weight data include every participant with a baseline and a month 6 or month 12 weight value, as appropriate
Similar articles
- Population profile and glycemic control following initiation or switch of injectable therapies in Tianjin, China: A real-world retrospective cohort study of adults with type 2 diabetes.
Zhang Q, Fan Y, Liu X, Zhang M, Zhang J, Du Q, Kang L, Chen L. Zhang Q, et al. J Diabetes Investig. 2025 May;16(5):842-851. doi: 10.1111/jdi.14415. Epub 2025 Feb 3. J Diabetes Investig. 2025. PMID: 39899440 Free PMC article. - A Real-World Observational Study Evaluating the Probability of Glycemic Control with Basal Insulin or Glucagon-Like Peptide-1 Receptor Agonist in Japanese Patients with Type 2 Diabetes.
Baxter M, Morimoto Y, Tamiwa M, Hattori M, Peng XV, Lubwama R, Maegawa H. Baxter M, et al. Diabetes Ther. 2020 Jul;11(7):1481-1496. doi: 10.1007/s13300-020-00836-8. Epub 2020 May 22. Diabetes Ther. 2020. PMID: 32445125 Free PMC article. - Titratable fixed-ratio combination of basal insulin plus a glucagon-like peptide-1 receptor agonist: A novel, simplified alternative to premix insulin for type 2 diabetes.
Gomez-Peralta F, Al-Ozairi E, Jude EB, Li X, Rosenstock J. Gomez-Peralta F, et al. Diabetes Obes Metab. 2021 Jul;23(7):1445-1452. doi: 10.1111/dom.14365. Epub 2021 Mar 31. Diabetes Obes Metab. 2021. PMID: 33651460 Free PMC article. Review. - Glycaemic and non-glycaemic efficacy of once-weekly GLP-1 receptor agonists in people with type 2 diabetes.
Patel D. Patel D. J Clin Pharm Ther. 2020 Sep;45 Suppl 1(Suppl 1):28-42. doi: 10.1111/jcpt.13224. J Clin Pharm Ther. 2020. PMID: 32910489 Free PMC article. Review.
Cited by
- Rationale for the Use of Combination Injectable Therapy in Patients With Type 2 Diabetes Who Have High A1C (≥9%) and/or Long Duration (>8 Years): Executive Summary.
Fonseca VA, Sood M, Galindo RJ. Fonseca VA, et al. Clin Diabetes. 2021 Apr;39(2):141-145. doi: 10.2337/cd20-0121. Clin Diabetes. 2021. PMID: 33986566 Free PMC article. No abstract available. - Simultaneous Versus Sequential Initiation of Lixisenatide and Basal Insulin for Type 2 Diabetes: Subgroup Analysis of a Japanese Post-Marketing Surveillance Study of Lixisenatide (PRANDIAL).
Kaneto H, Baxter M, Takahashi Y, Terauchi Y. Kaneto H, et al. Adv Ther. 2022 Dec;39(12):5453-5473. doi: 10.1007/s12325-022-02311-1. Epub 2022 Oct 7. Adv Ther. 2022. PMID: 36207508 Free PMC article. - Translating iGlarLixi Evidence for the Management of Frequent Clinical Scenarios in Type 2 Diabetes.
Skolnik N, Del Prato S, Blonde L, Galstyan G, Rosenstock J. Skolnik N, et al. Adv Ther. 2021 Apr;38(4):1715-1731. doi: 10.1007/s12325-020-01614-5. Epub 2021 Feb 23. Adv Ther. 2021. PMID: 33620694 Free PMC article. Review. - Population profile and glycemic control following initiation or switch of injectable therapies in Tianjin, China: A real-world retrospective cohort study of adults with type 2 diabetes.
Zhang Q, Fan Y, Liu X, Zhang M, Zhang J, Du Q, Kang L, Chen L. Zhang Q, et al. J Diabetes Investig. 2025 May;16(5):842-851. doi: 10.1111/jdi.14415. Epub 2025 Feb 3. J Diabetes Investig. 2025. PMID: 39899440 Free PMC article. - Treatment satisfaction and time in range after 16 weeks of treatment with iGlarLixi in insulin-naive adults with suboptimally controlled type 2 diabetes.
Shah VN, Dex T, Meneghini L, Rodrigues A, Polonsky WH. Shah VN, et al. Diabetes Obes Metab. 2025 May;27(5):2523-2530. doi: 10.1111/dom.16251. Epub 2025 Feb 14. Diabetes Obes Metab. 2025. PMID: 39950217 Free PMC article. Clinical Trial.
References
- Davies M, D'Alessio D, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461‐2498. - PubMed
- American Diabetes Association . Standards of medical care in diabetes ‐ 2020. Diabetes Care. 2020;43(Suppl 1):S1‐S213. - PubMed
- Peng X, Blonde L, Shepherd L, Lubwama R, Ji L, McCrimmon R. A real‐world retrospective study evaluating glycaemic control with glucagon‐like peptide‐1 receptor agonists or basal insulin in type 2 diabetes in the UK. Diabetologia. 2019;62:S420‐S421.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical