Insights into the evolution of regulated actin dynamics via characterization of primitive gelsolin/cofilin proteins from Asgard archaea - PubMed (original) (raw)
Insights into the evolution of regulated actin dynamics via characterization of primitive gelsolin/cofilin proteins from Asgard archaea
Caner Akıl et al. Proc Natl Acad Sci U S A. 2020.
Abstract
Asgard archaea genomes contain potential eukaryotic-like genes that provide intriguing insight for the evolution of eukaryotes. The eukaryotic actin polymerization/depolymerization cycle is critical for providing force and structure in many processes, including membrane remodeling. In general, Asgard genomes encode two classes of actin-regulating proteins from sequence analysis, profilins and gelsolins. Asgard profilins were demonstrated to regulate actin filament nucleation. Here, we identify actin filament severing, capping, annealing and bundling, and monomer sequestration activities by gelsolin proteins from Thorarchaeota (Thor), which complete a eukaryotic-like actin depolymerization cycle, and indicate complex actin cytoskeleton regulation in Asgard organisms. Thor gelsolins have homologs in other Asgard archaea and comprise one or two copies of the prototypical gelsolin domain. This appears to be a record of an initial preeukaryotic gene duplication event, since eukaryotic gelsolins are generally comprise three to six domains. X-ray structures of these proteins in complex with mammalian actin revealed similar interactions to the first domain of human gelsolin or cofilin with actin. Asgard two-domain, but not one-domain, gelsolins contain calcium-binding sites, which is manifested in calcium-controlled activities. Expression of two-domain gelsolins in mammalian cells enhanced actin filament disassembly on ionomycin-triggered calcium release. This functional demonstration, at the cellular level, provides evidence for a calcium-controlled Asgard actin cytoskeleton, indicating that the calcium-regulated actin cytoskeleton predates eukaryotes. In eukaryotes, dynamic bundled actin filaments are responsible for shaping filopodia and microvilli. By correlation, we hypothesize that the formation of the protrusions observed from Lokiarchaeota cell bodies may involve the gelsolin-regulated actin structures.
Keywords: Asgard archaea; X-ray crystallography; actin; eukaryogenesis; gelsolin.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures
Fig. 1.
Thor gelsolins and actin regulation. (A) Schematic representation of the three Thor gelsolin architectures and the hypothetical evolution of the gelsolin family. Ovals depict gelsolin domains. Ticks indicate potential calcium-binding residues and red triangles denote a central WH2-like motif. Since Type II calcium-binding sites (48) (red ticks) are found in both domains of 2DGel, a calcium-binding single-domain protein likely existed in evolution that is not found in the current sequence databases. This is indicated by the “proposed calcium-binding intermediates.” The Type I site (48) (gray ticks) may have been present in this proposed calcium-binding intermediate, and later lost from domain two after the first gene duplication. Alternatively, the Type I site may have appeared in domain one after the first gene duplication. The architectures of typical eukaryotic gelsolin-like proteins are included for comparison. (B_–_E) Pyrene-actin polymerization profiles of 2 μM actin (blue) supplemented with (B) 1DGelX (1 mM EGTA), at 10 nM (red), 0.1 μM (green), or 2 μM (fawn) or 16 μM (dark brown), (C) supplemented with 5 nM (red), 0.05 μM (green), 2 μM (fawn) human gelsolin (0.3 mM CaCl2), or supplemented with (D) 2DGel (1 mM EGTA) or (E) 2DGel (1.0 mM CaCl2) at the concentrations in B. (F_–_I) Actin depolymerization profiles of 2 μM actin (blue), supplemented by (F) 1DGelX (1 mM EGTA), at 2 μM (red), 8 μM (lilac), or 32 μM (black), (G) human gelsolin in 0.3 mM CaCl2, concentrations as in C, (H) 2DGel (0.3 mM EGTA) or (I) 2DGel (1 mM CaCl2) at the concentrations in F. Two other 2DGel orthologs, 2DGel2 and 2DGel3, showed additional filament nucleation activity and more potent severing activity. All three 2DGel proteins were less active at 0.3 mM than at 1 mM Ca2+, and inactive in 1 mM EGTA in terms of severing activity (
SI Appendix, Fig. S2 E_–_P
). (J) Pyrene-actin polymerization profiles of 2 μM actin (blue) supplemented with 2 μM (fawn), 8 μM (lilac), 32 μM (dark green), or 128 μM (light green) ProGel in 0.3 mM Ca2+. (K) Pyrene-actin depolymerization profiles of 2 μM actin (blue) supplemented with 10 nM (red), 0.1 μM (green), 2 μM (beige), or 16 μM (dark brown) ProGel in 0.3 mM CaCl2. (L) SDS/PAGE analysis of actin filaments (8 μM) in the presence or absence of ProGel (256 μM). At 150,000 × g (high) filaments were pelleted in both conditions, whereas at 10,000 × g (low) actin was pelleted as bundles only in the presence of ProGel.
Fig. 2.
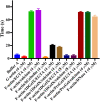
F-actin severing by 1DGelX. Viscometry experiments demonstrate that 4 μM ProGel or DNase I (actin-sequestering protein control) do not significantly change the viscosity of F-actin (∼70 s) in the timeframe of the experiment, whereas, 4- and 8 μM 1DGelX lowers the F-actin viscosity levels close to G-actin (∼5 s) or to gelsolin (F-actin severing protein control). x axis indicates solution conditions.
Fig. 3.
The regulation of actin assembly and disassembly by Thor gelsolins followed by TIRF microscopy. Time course of the (A) assembly and (B) disassembly of 1.5 μM actin in the presence of various concentrations of Thor gelsolins. (Scale bar, 20 μm.) Titrations of ProGel and 1DGelX in the assembly assay can be found in
SI Appendix, Fig. S5
, and comparison of 2DGel, 2DGel2, and 2DGel3 in the disassembly assay are found in
SI Appendix, Fig. S6
. Movies of the assembly/disassembly of Thor gelsolins are found in
Movies S1–S5
. ProGel and 1DGelX assays were carried out in 1 mM EGTA. The 2DGel assembly assays were in 0.3 mM CaCl2 or 1 mM EGTA, and the disassembly assay in 0.3 mM CaCl2.
Fig. 4.
The structures of ProGel and 2DGel in complex with rActin. (A) The ProGel/rActin complex. rActin is shown as a surface and ProGel is in schematic representation. (B) The structure of the 2DGel/rActin complex. The four calcium ions associated with 2DGel are shown as black spheres. The crystal structure of 2DGel3/rActin complex is found in
SI Appendix, Fig. S7_A_
. (C) The structure of the first three domains of human gelsolin in complex with rActin for comparison (PDB ID code 1EQY). (D_–_G) Side views of (D) ProGel, (E) twinfilin domain 2 (D2), a cofilin-family member (PDB ID code 3DAW), (F) 2DGel domain 1 (D1), and (G) gelsolin domain G1, in complex with actin. Actin is shown as a trace. Similar representations for 2DGel3 is found in
SI Appendix, Fig. S7_B_
. The arrow indicates the displacement of ProGel relative to G1. Data collection and refinement statistics are found in
SI Appendix, Table S1
. (H) Structure-based sequence alignment of the core region of the gelsolin/cofilin domain of ProGel, 2DGel, Sec23a (which has not been shown to bind actin), hGelsolin, and cofilin. rActin interacting residues are indicated by stars. Asgard proteins above the alignment ProGel, red; 2DGel, green) and human proteins below (gelsolin, blue; cofilin, orange). The consensus secondary structure is shown in black.
Fig. 5.
Structural homology to gelsolin and cofilin. (A) The three conserved calcium-binding sites in 2DGel and 2DGel3 are shared with the first two domains of human gelsolin. (B) Structural comparisons and superimpositions of ProGel with human gelsolin G1 (PDB ID code 3FFN) and cofilin (PDB ID code 4KEE). Calcium ions are shown as lime or black spheres. Asterisks indicate additional helices in the cofilin fold relative to ProGel.
Fig. 6.
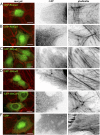
Localization of GFP-Thor proteins in mammalian cells. Ectopically expressed Asgard gelsolins in human U2OS cells followed by fluorescence imaging. (A and B) Two examples of merged images of GFP-ProGel (green) and rhodamine-phalloidin staining of actin filament structures (red), followed by enlargements of the box regions with separated GFP and phalloidin channels. Similar representative images of (C) GFP-2DGel, (D) GFP-2DGel2, (E) GFP-1DGelX, and (F) GFP control.
Fig. 7.
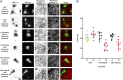
Calcium signaling to ectopically expressed 2DGel proteins in human U2OS cells followed by fluorescence imaging. (A) Cells expressing GFP, GFP-2DGel, or GFP-2DGel2 are indicated by signal the GFP channel, columns 1 and 2, and highlighted with asterisks. Actin filaments and larger structures are observed in the rhodamine-phalloidin channel, columns 3 and 4. “+ve” refers to normal and “−ve” to the reversed image. Merged images of the GFP channel (green) and the rhodamine-phalloidin channel (red) are in the final column. Different cells were imaged immediately before, or 10 min after, treatment with ionomycin to release calcium. (B) Quantification of rhodamine-phalloidin fluorescence before and 10 min after treatment with ionomycin. Twelve-bit monochrome images of actin fluorescence intensity, of typical cells, were quantified as a ratio for GFP and adjacent non-GFP expressing cells, in three separate experiments.
Similar articles
- Structural and biochemical evidence for the emergence of a calcium-regulated actin cytoskeleton prior to eukaryogenesis.
Akıl C, Tran LT, Orhant-Prioux M, Baskaran Y, Senju Y, Takeda S, Chotchuang P, Muengsaen D, Schulte A, Manser E, Blanchoin L, Robinson RC. Akıl C, et al. Commun Biol. 2022 Aug 31;5(1):890. doi: 10.1038/s42003-022-03783-1. Commun Biol. 2022. PMID: 36045281 Free PMC article. - Genomes of Asgard archaea encode profilins that regulate actin.
Akıl C, Robinson RC. Akıl C, et al. Nature. 2018 Oct;562(7727):439-443. doi: 10.1038/s41586-018-0548-6. Epub 2018 Oct 3. Nature. 2018. PMID: 30283132 - Mythical origins of the actin cytoskeleton.
Akıl C, Kitaoku Y, Tran LT, Liebl D, Choe H, Muengsaen D, Suginta W, Schulte A, Robinson RC. Akıl C, et al. Curr Opin Cell Biol. 2021 Feb;68:55-63. doi: 10.1016/j.ceb.2020.08.011. Epub 2020 Oct 10. Curr Opin Cell Biol. 2021. PMID: 33049465 Review. - Arabidopsis VILLIN1 generates actin filament cables that are resistant to depolymerization.
Huang S, Robinson RC, Gao LY, Matsumoto T, Brunet A, Blanchoin L, Staiger CJ. Huang S, et al. Plant Cell. 2005 Feb;17(2):486-501. doi: 10.1105/tpc.104.028555. Epub 2005 Jan 19. Plant Cell. 2005. PMID: 15659626 Free PMC article. - Archaeal Actin-Family Filament Systems.
Lindås AC, Valegård K, Ettema TJG. Lindås AC, et al. Subcell Biochem. 2017;84:379-392. doi: 10.1007/978-3-319-53047-5_13. Subcell Biochem. 2017. PMID: 28500533 Review.
Cited by
- Expanded diversity of Asgard archaea and their relationships with eukaryotes.
Liu Y, Makarova KS, Huang WC, Wolf YI, Nikolskaya AN, Zhang X, Cai M, Zhang CJ, Xu W, Luo Z, Cheng L, Koonin EV, Li M. Liu Y, et al. Nature. 2021 May;593(7860):553-557. doi: 10.1038/s41586-021-03494-3. Epub 2021 Apr 28. Nature. 2021. PMID: 33911286 Free PMC article. - Endosymbiotic selective pressure at the origin of eukaryotic cell biology.
Raval PK, Garg SG, Gould SB. Raval PK, et al. Elife. 2022 Nov 10;11:e81033. doi: 10.7554/eLife.81033. Elife. 2022. PMID: 36355038 Free PMC article. Review. - The evolution and diversity of actin-dependent cell migration.
Fritz-Laylin LK, Titus MA. Fritz-Laylin LK, et al. Mol Biol Cell. 2023 Nov 1;34(12):pe6. doi: 10.1091/mbc.E22-08-0358. Mol Biol Cell. 2023. PMID: 37906436 Free PMC article. Review. - Heimdallarchaea encodes profilin with eukaryotic-like actin regulation and polyproline binding.
Survery S, Hurtig F, Haq SR, Eriksson J, Guy L, Rosengren KJ, Lindås AC, Chi CN. Survery S, et al. Commun Biol. 2021 Sep 1;4(1):1024. doi: 10.1038/s42003-021-02543-x. Commun Biol. 2021. PMID: 34471213 Free PMC article. - Structural and biochemical evidence for the emergence of a calcium-regulated actin cytoskeleton prior to eukaryogenesis.
Akıl C, Tran LT, Orhant-Prioux M, Baskaran Y, Senju Y, Takeda S, Chotchuang P, Muengsaen D, Schulte A, Manser E, Blanchoin L, Robinson RC. Akıl C, et al. Commun Biol. 2022 Aug 31;5(1):890. doi: 10.1038/s42003-022-03783-1. Commun Biol. 2022. PMID: 36045281 Free PMC article.
References
- Zaremba-Niedzwiedzka K. et al. ., Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 541, 353–358 (2017). - PubMed
- Akıl C., Robinson R. C., Genomes of Asgard archaea encode profilins that regulate actin. Nature 562, 439–443 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials