BIN2 orchestrates platelet calcium signaling in thrombosis and thrombo-inflammation - PubMed (original) (raw)
. 2020 Nov 2;130(11):6064-6079.
doi: 10.1172/JCI136457.
Charly Kusch 1 2, Sarah Beck 1 2, Michael Popp 1 2, Timo Vögtle 1 2, Mara Meub 3, Inga Scheller 1 2, Hannah S Heil 2, Julia Preu 1 2, Michael K Schuhmann 4, Katherina Hemmen 2, Thomas Premsler 5, Albert Sickmann 5 6 7, Katrin G Heinze 2, David Stegner 1 2, Guido Stoll 4, Attila Braun 1 2, Markus Sauer 3, Bernhard Nieswandt 1 2
Affiliations
- PMID: 32750041
- PMCID: PMC7598067
- DOI: 10.1172/JCI136457
BIN2 orchestrates platelet calcium signaling in thrombosis and thrombo-inflammation
Julia Volz et al. J Clin Invest. 2020.
Abstract
Store-operated Ca2+ entry (SOCE) is the major route of Ca2+ influx in platelets. The Ca2+ sensor stromal interaction molecule 1 (STIM1) triggers SOCE by forming punctate structures with the Ca2+ channel Orai1 and the inositol trisphosphate receptor (IP3R), thereby linking the endo-/sarcoplasmic reticulum to the plasma membrane. Here, we identified the BAR domain superfamily member bridging integrator 2 (BIN2) as an interaction partner of STIM1 and IP3R in platelets. Deletion of platelet BIN2 (Bin2fl/fl,Pf4-Cre mice) resulted in reduced Ca2+ store release and Ca2+ influx in response to all tested platelet agonists. These defects were a consequence of impaired IP3R function in combination with defective STIM1-mediated SOC channel activation, while Ca2+ store content and agonist-induced IP3 production were unaltered. This severely defective Ca2+ signaling translated into impaired thrombus formation under flow and a protection of Bin2fl/fl,Pf4-Cre mice in models of arterial thrombosis and stroke. Our results establish BIN2 as a central regulator of platelet activation in thrombosis and thrombo-inflammatory disease settings.
Keywords: Calcium signaling; Cell Biology; Hematology; Platelets; Thrombosis.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures
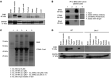
Figure 1. BIN2 is highly expressed in human and mouse platelets.
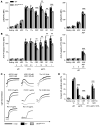
(A) Western blot analysis of BIN2 expression in human tissue lysates; GAPDH was used as loading control. (B) IP of BIN2 was performed with human platelet lysates using an anti-BIN2 antibody. BIN2, STIM1, and RhoA were detected by Western blot. Representative result of 3 independent experiments. Lane 4 was run on the same gel as 1 to 3, but the lanes were noncontiguous. (C) Recombinant GST-tagged STIM1 C-tail incubated with recombinant BIN2 protein. Pulldown of the GST-tagged STIM1 was performed, and the resulting fractions were analyzed by SDS page and silver staining. (D) Western blot analysis of BIN2 expression in the indicated tissue lysates of WT and Bin2–/– mice with GAPDH as loading control. See complete unedited blots in the supplemental material.
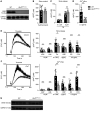
Figure 2. Impaired Ca2+ store release and influx in Bin2fl/fl,Pf4Cre platelets.
(A) Western blot analysis of BIN2 expression in WT and Bin2fl/fl,Pf4Cre platelet lysates with GAPDH as loading control. (B) Ca2+ store content was measured upon stimulation with 5 μM ionomycin in the presence of 0.5 mM EGTA (n = 10). (C) Ca2+ store release was measured in the absence of extracellular Ca2+ upon treatment with 0.1 μM or 5 μM thapsigargin (TG) (n ≥ 8) and (D) Ca2+ influx upon treatment with 0.1 μM TG (n = 8). (E and F) Representative traces and statistical analysis of store release (E) and Ca2+ influx (F) upon activation of the platelets with the indicated agonists (n ≥ 8). (G) Western blot analysis of WT and Bin2fl/fl,Pf4Cre platelet lysates detecting the expression of STIM1 with GAPDH as loading control. Values are depicted as mean ± SD, and P values were calculated using the Mann-Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001. See complete unedited blots in the supplemental material.
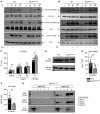
Figure 3. Defective IP3R function in Bin2fl/fl,Pf4Cre mice and BIN2 interaction with STIM1 and IP3R.
(A and B) Determination of whole-cell tyrosine phosphorylation pattern in WT and Bin2fl/fl,Pf4Cre platelets upon activation with convulxin (CVX) (A) or rhodocytin (RC) (B). The samples were taken at the indicated time points, lysed, and Western blot analysis was performed with the indicated phospho-specific and pan-antibodies. This blot is representative of 3 independent experiments. (C) Quantification of inositol monophosphate (IP1), a specific metabolite of inositol-1,4,5-trisphosphate (IP3), produced upon activation with the indicated agonist (n = 3). Representative results of 3 independent experiments. (D) Western blot analysis of IP3R expression in WT and Bin2fl/fl,Pf4Cre platelet lysates; GAPDH was used as loading control. (E and F) Ca2+ concentrations in the cytoplasm of WT and Bin2fl/fl,Pf4Cre platelets upon treatment with UV light–inducible IP3 in the (E) absence or (F) presence of extracellular Ca2+ (n = 8). (G) Bin2fl/fl,Pf4Cre platelet lysates were incubated with recombinant BIN2-HIS protein, followed by a purification step with NI-NTA beads. The different fractions were eluted and analyzed by Western blotting using BIN2-, STIM1-, and IP3R-specific antibodies. Representative result of 3 independent experiments. Values are depicted as mean ± SD, and P values were calculated using the Mann-Whitney U test. **P < 0.01. See complete unedited blots in the supplemental material.
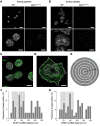
Figure 4. BIN2 STIM1 colocalization in human platelets.
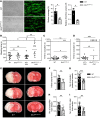
_d_STORM images of (A) resting mouse platelets on glycine or (B) thrombin-activated WT and Bin2fl/fl,Pf4Cre mouse platelets on fibrinogen, stained for BIN2. Scale bars 5 μm (upper panel) and 2 μm (lower panel). Dual-color _d_STORM allows resolution of the distribution of STIM1 (magenta) and BIN2 (green) in (C) resting and (D) activated human platelets; dashed white lines indicate areas of neighbor density analysis (E) between STIM1 (magenta) and BIN2 (green), with discrete ring areas in which the BIN2 density was determined (dark gray). In activated spread platelets, only localizations in the inner region where the organelles and intercellular membrane compartments accumulate were considered for STIM1. Colocalization hotspots of STIM1 and BIN2 are identified by analyzing the BIN2 density at increasing radial distances to STIM1: Histograms of the distance-dependent BIN2 density peak maxima found for (F) resting (n = 6, Supplemental Figure 7A) and (G) activated human platelets (n = 14, Supplemental Figure 7B). The distinct STIM1 to BIN2 distance regions with increased accumulation of colocalization hotspots are highlighted in light gray. Scale bars (C and D): 2 μm.
Figure 5. Defective integrin activation and aggregation of Bin2fl/fl,Pf4Cre platelets upon (hem)ITAM stimulation.
(A and B) Flow cytometric analysis of (A) integrin αIIbβ3 activation (binding of JON/A-PE) and (B) degranulation-dependent P-selectin exposure in WT and Bin2fl/fl,Pf4Cre platelets in response to the indicated agonists (n = 4). Representative result of 3 independent experiments. Values are depicted as mean ± SD, and P values were calculated using the Mann-Whitney U test. **P < 0.01, ***P < 0.001. (C) Washed platelets were stimulated with the indicated agonists, and light transmission was recorded using a 4-channel aggregometer. Representative results of 3 independent experiments. (D) Phosphatidylserine exposure of platelets in response to the indicated agonists was measured by flow cytometry. n ≥ 5. Representative result of 3 independent experiments. Values are depicted as mean ± SD, and P values were calculated using the Mann-Whitney U test. **P < 0.01, ***P < 0.001.
Figure 6. Impaired hemostasis, defective arterial thrombus formation, and protection from ischemic brain infarction in Bin2fl/fl,Pf4Cre mice.
(A) Adhesion and thrombus formation of platelets on collagen was assessed in a flow adhesion assay at a wall shear rate of 1700/s. Representative images and the quantification of the surface coverage and the relative thrombus volume are shown (n = 4). Representative example of 3 independent experiments. Values are depicted as mean ± SD, and P values were calculated using the Mann-Whitney U test. *P < 0.05. Scale bar: 50 μm. (B) Tail bleeding times of WT and Bin2fl/fl,Pf4Cre mice with or without acetylsalicylic acid (ASA) treatment (1 mg/kg i.v.) 15 minutes before the start of the experiment. Each symbol represents 1 animal. Kruskal-Wallis test (P value: 0.0022) followed by Dunn’s multiple-comparisons post hoc test; *P < 0.05, **P < 0.01. (C and D) Time to stable vessel occlusion of WT and Bin2fl/fl,Pf4Cre mice without (C) or with (D) ASA treatment (1 mg/kg i.v.) 15 minutes before the start of the experiment. The abdominal aorta was injured by firm compression with forceps, and blood flow was monitored for 30 minutes. Mann-Whitney U test was performed to compare mean occlusion times of occluded vessels. To compare frequency of occluded and nonoccluded vessels, Fisher’s exact test was used. *P < 0.05, ***P < 0.001. (E–I) WT and Bin2fl/fl Pf4-Cre mice were subjected to 60 minutes of transient middle cerebral artery occlusion (tMCAO) n = 10. (E) Representative images of 3 coronal brain sections stained with TTC. (F) Infarct volumes, (G) number of occluded ipsilateral vessels (2 slides per animal analyzed), (H) number of neutrophils, and (I) CD11b+ cells per section (2 slides per animal analyzed) were investigated after 24 hours. Values are depicted as mean ± SD, and P values were calculated using the Mann-Whitney U test. **P < 0.01, ***P < 0.001.
Similar articles
- Suppressed ORAI1-STIM1-dependent Ca2+ entry by protein kinase C isoforms regulating platelet procoagulant activity.
Zou J, Zhang P, Solari FA, Schönichen C, Provenzale I, Mattheij NJA, Kuijpers MJE, Rauch JS, Swieringa F, Sickmann A, Zieger B, Jurk K, Heemskerk JWM. Zou J, et al. J Biol Chem. 2024 Dec;300(12):107899. doi: 10.1016/j.jbc.2024.107899. Epub 2024 Oct 17. J Biol Chem. 2024. PMID: 39424145 Free PMC article. - Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation.
Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bösl M, Stoll G, Nieswandt B. Braun A, et al. Blood. 2009 Feb 26;113(9):2056-63. doi: 10.1182/blood-2008-07-171611. Epub 2008 Oct 2. Blood. 2009. PMID: 18832659 - 1,25(OH)2 vitamin D3-dependent inhibition of platelet Ca2+ signaling and thrombus formation in klotho-deficient mice.
Borst O, Münzer P, Schmid E, Schmidt EM, Russo A, Walker B, Yang W, Leibrock C, Szteyn K, Schmidt S, Elvers M, Faggio C, Shumilina E, Kuro-o M, Gawaz M, Lang F. Borst O, et al. FASEB J. 2014 May;28(5):2108-19. doi: 10.1096/fj.13-239277. Epub 2014 Feb 12. FASEB J. 2014. PMID: 24522202 - Store-operated calcium entry in thrombosis and thrombo-inflammation.
Mammadova-Bach E, Nagy M, Heemskerk JWM, Nieswandt B, Braun A. Mammadova-Bach E, et al. Cell Calcium. 2019 Jan;77:39-48. doi: 10.1016/j.ceca.2018.11.005. Epub 2018 Nov 23. Cell Calcium. 2019. PMID: 30530092 Review. - Calcium signaling in platelets.
Varga-Szabo D, Braun A, Nieswandt B. Varga-Szabo D, et al. J Thromb Haemost. 2009 Jul;7(7):1057-66. doi: 10.1111/j.1538-7836.2009.03455.x. Epub 2009 Apr 24. J Thromb Haemost. 2009. PMID: 19422456 Review.
Cited by
- Challenges and Improvements of Novel Therapies for Ischemic Stroke.
Yang L, Qian J, Yang B, He Q, Wang J, Weng Q. Yang L, et al. Front Pharmacol. 2021 Sep 30;12:721156. doi: 10.3389/fphar.2021.721156. eCollection 2021. Front Pharmacol. 2021. PMID: 34658860 Free PMC article. Review. - Plasma Proteomic Signature Predicts Myeloid Neoplasm Risk.
Tran D, Beeler JS, Liu J, Wiley B, Chan ICC, Xin Z, Kramer MH, Batchi-Bouyou AL, Zong X, Walter MJ, Petrone GEM, Chlamydas S, Ferraro F, Oh ST, Link DC, Busby B, Cao Y, Bolton KL. Tran D, et al. Clin Cancer Res. 2024 Aug 1;30(15):3220-3228. doi: 10.1158/1078-0432.CCR-23-3468. Clin Cancer Res. 2024. PMID: 38446993 Free PMC article. - Identification of the Antithrombotic Mechanism of Leonurine in Adrenalin Hydrochloride-Induced Thrombosis in Zebrafish via Regulating Oxidative Stress and Coagulation Cascade.
Liao L, Zhou M, Wang J, Xue X, Deng Y, Zhao X, Peng C, Li Y. Liao L, et al. Front Pharmacol. 2021 Nov 4;12:742954. doi: 10.3389/fphar.2021.742954. eCollection 2021. Front Pharmacol. 2021. PMID: 34803688 Free PMC article. - Suppressed ORAI1-STIM1-dependent Ca2+ entry by protein kinase C isoforms regulating platelet procoagulant activity.
Zou J, Zhang P, Solari FA, Schönichen C, Provenzale I, Mattheij NJA, Kuijpers MJE, Rauch JS, Swieringa F, Sickmann A, Zieger B, Jurk K, Heemskerk JWM. Zou J, et al. J Biol Chem. 2024 Dec;300(12):107899. doi: 10.1016/j.jbc.2024.107899. Epub 2024 Oct 17. J Biol Chem. 2024. PMID: 39424145 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous