The natural function of the malaria parasite's chloroquine resistance transporter - PubMed (original) (raw)
The natural function of the malaria parasite's chloroquine resistance transporter
Sarah H Shafik et al. Nat Commun. 2020.
Abstract
The Plasmodium falciparum chloroquine resistance transporter (PfCRT) is a key contributor to multidrug resistance and is also essential for the survival of the malaria parasite, yet its natural function remains unresolved. We identify host-derived peptides of 4-11 residues, varying in both charge and composition, as the substrates of PfCRT in vitro and in situ, and show that PfCRT does not mediate the non-specific transport of other metabolites and/or ions. We find that drug-resistance-conferring mutations reduce both the peptide transport capacity and substrate range of PfCRT, explaining the impaired fitness of drug-resistant parasites. Our results indicate that PfCRT transports peptides from the lumen of the parasite's digestive vacuole to the cytosol, thereby providing a source of amino acids for parasite metabolism and preventing osmotic stress of this organelle. The resolution of PfCRT's native substrates will aid the development of drugs that target PfCRT and/or restore the efficacy of existing antimalarials.
Conflict of interest statement
The authors declare no competing interests.
Figures
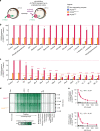
Fig. 1. Screening of a solute library for _cis_-inhibitory activity identified potential substrates of PfCRT.
a Schematic showing the _cis_-inhibition of [3H]CQ uptake via PfCRTDd2 or PfCRTEcu1110 by an unlabelled solute in the Xenopus oocyte system. The library included solutes known to exist in the parasitised erythrocyte, such as vitamins, carbohydrates, metal ions, lipids, amino acids, peptides and other organic and inorganic molecules. b, c The effect on [3H]CQ transport via PfCRT by a subset of solutes (2 mM) (b) or host-derived peptides (2 mM) (c). Non-expressing oocytes and those expressing PfCRT3D7 were included as negative controls; these oocytes accumulate a low level of [3H]CQ via simple diffusion of the neutral species,, reflecting the background level of [3H]CQ transport. Note that aspartate and vitamins E and B6 caused modest reductions in PfCRTDd2-mediated transport, but only aspartate was found to inhibit PfCRTEcu1110. A diverse range of peptides cause potent inhibition of both PfCRTDd2 or PfCRTEcu1110. d Heatmap of the _cis_-inhibition of [3H]CQ transport via PfCRTDd2 and PfCRTEcu1110 by 173 different solutes. e VF-6 (top) and HM-5 (bottom) inhibit [3H]CQ transport via PfCRTDd2 in a concentration-dependent manner. The half-maximum inhibitory concentrations (IC50) for VF-6 and HM-5 are 207 ± 12 and 167 ± 14 µM, respectively. The data are the mean of n = 4 independent experiments (each yielding similar results and overlaid as individual data points in b and c), and the error is the SEM. Where not visible, the error bars fall within the symbols. The asterisks denote a significant difference from the relevant PfCRTDd2 (red asterisks) or PfCRTEcu1110 (orange asterisks) control; *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA). The source datasets are provided as a Source Data file.
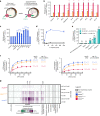
Fig. 2. PfCRT is _trans_-stimulated by peptides containing 4–11 amino acid residues.
a Schematic showing the _trans-_stimulation of [3H]CQ transport via PfCRT by an unlabelled substrate in Xenopus oocytes. b, c_Trans_-stimulation of [3H]CQ transport via PfCRTDd2 or PfCRTEcu1110 (b) or via PfCRT3D7 (c) by a subset of host-derived peptides and peptide mimics (45 mM). The test solute was injected into the oocyte immediately prior to the commencement of the assay, with the buffer-only and LH treatments serving as injection controls. The negative controls were non-expressing oocytes and those expressing an unrelated P. falciparum transporter, the nucleoside transporter 1 (PfNT1,). The latter demonstrates that the expression of a similarly sized transporter in Xenopus oocytes does not affect the ability of the oocyte membrane to reseal following injection of a test solute. d_Trans_-stimulation of [3H]CQ transport via PfCRT3D7 by VF-6 (45 mM) is pH-dependent, with the largest increase observed when the pH of the injection buffer was 5.5 (equating to 79 fmoles H+). e_Trans_-stimulation of [3H]CQ transport via PfCRT is unaffected by the removal of Na+ from the injection buffer. f Concentration-dependence of the _trans_-stimulation of [3H]CQ transport via PfCRT by VF-6 (left) and HM-5 (right). The non-expressing oocyte data overlays the data obtained with oocytes expressing PfNT1. g Heatmap of the _trans_-stimulation of [3H]CQ transport via PfCRT3D7, PfCRTEcu1110 or PfCRTDd2 by 173 different solutes. Only peptides and peptide mimics _trans_-stimulate PfCRT; all other solutes, including aspartate and vitamins E and B6, failed to increase [3H]CQ transport via PfCRT are thus unlikely to be substrates of the transporter. The data are the mean of n = 4 independent experiments (each yielding similar results and overlaid as individual data points in b, c and e), and the error is the SEM. Where not visible, the error bars fall within the symbols. The asterisks denote a significant difference from the relevant PfCRTDd2 (red asterisks), PfCRTEcu1110 (orange asterisks) or PfCRT3D7 (blue asterisks) buffer-injected control; *P < 0.05, **P < 0.01, ***P < 0.001, ns: not significant (one-way ANOVA). SQV: saquinavir. The source datasets are provided as a Source Data file.
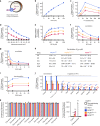
Fig. 3. Characterisation of the transport of a host-derived hexapeptide via PfCRT.
a Schematic showing the transport of [3H]VF-6 via PfCRT in Xenopus oocytes. b–e [3H]VF-6 transport via PfCRT is approximately linear with time for at least 2 h (b), is dependent on both the pH of the injection buffer (the highest level of transport occurred at pH 5.5, equating to 79 fmoles H+) (c) and the membrane potential (with the rate of VF-6 efflux steadily decreasing as the membrane potential became more positive) (d), and is saturable (PfCRT3D7 possesses a slightly lower affinity and higher _V_max relative to the mutant transporters) (e). [3H]CQ influx is also dependent on the membrane potential (Supplementary Fig. 2g and Supplementary Note 3). f, g_Cis_-inhibition of [3H]VF-6 transport via PfCRT by verapamil (VP) (f) and Q2C (g). h The half-maximum inhibitory concentrations (IC50s) for the _cis_-inhibition of [3H]VF-6 transport via PfCRT by drugs known to interact with the transporter. i_Trans_-stimulation of [3H]VF-6 transport via PfCRT by saquinavir (SQV). j_Trans_-inhibition of [3H]VF-6 transport via PfCRT by haemoglobin-peptides (45 mM). k Left: various other radiolabelled solutes are not transported out of the oocyte by PfCRT. Right: in the same assay, [3H]CQ is effluxed via PfCRTEcu1110 and PfCRTDd2 and [3H]hypoxanthine is effluxed by PfNT1. The data are the mean of n = 5 independent experiments (each yielding similar results and overlaid as individual data points in j and k), and the error is the SEM. Where not visible, the error bars fall within the symbols. The non-expressing oocyte data overlays the data obtained with oocytes expressing PfNT1 in b, c, e–g and i. The asterisks denote a significant difference from the relevant PfCRTDd2 (red asterisks), PfCRTEcu1110 (orange asterisks), PfCRT3D7 (blue asterisks) or non-expressing (black asterisks) control; *P < 0.05, **P < 0.01, ***P < 0.001, ns not significant (one-way ANOVA). The source datasets are provided as a Source Data file.
Fig. 4. Diverse field and laboratory-derived isoforms of PfCRT transport the VF-6 peptide.
a The VF-6 transport activities, including kinetic parameters, of various field and laboratory-derived PfCRT isoforms in Xenopus oocytes (_K_m, Michaelis–Menten constant; _V_max, maximum velocity). b The capacity of PfCRT to transport VF-6 decays exponentially as the protein’s CQ transport activity increases (_R_2 = 0.93). Exceptions to this trend include PfCRTCam734, L272F-PfCRTDd2, L272F-PfCRT3D7 and C101F-PfCRTDd2. c [3H]CQ transport (left) and [3H]VF-6 transport (right) via epitope-tagged versions of PfCRT3D7 and PfCRTDd2. The version of PfCRTDd2 carrying a C-terminal 3xmyc tag does not mediate [3H]CQ transport. The other four variants of PfCRTDd2 retain all or most of their CQ transport activity. By contrast, none of the tagged versions of PfCRT3D7 or PfCRTDd2 transport [3H]VF-6. The fusion of polypeptides to PfCRT can therefore abolish its ability to transport peptides, even when the protein remains able to transport CQ. The data are the mean of n = 5 independent experiments (each yielding similar results and overlaid as individual data points in a and c), and the error is the SEM. Where not visible, the error bars fall within the symbols. The asterisks denote a significant difference from the relevant PfCRTDd2 (red asterisks) or PfCRT3D7 (blue asterisks) control; *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA). The source datasets are provided as a Source Data file.
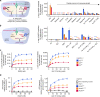
Fig. 5. Peptide mimics are substrates of PfCRT in situ.
a Left: schematic showing the H+-efflux assay. If a protonated solute is a substrate of PfCRT it will cause a H+ leak when effluxed from the DV. Inhibition of the DV’s H+-ATPase by concanamycin A enables this H+ leak to be detected (with a pH-sensitive probe) as an increase in the rate of DV alkalinisation. Right: several peptide mimics (10 μM) increase the rate of DV alkalinisation in C2GC03, C67G8 and C4Dd2 parasites. CQ (the positive control) causes the rate of DV alkalinisation to increase in the C67G8 and C4Dd2 lines and to decrease slightly in C2GC03 parasites. This is consistent with previous applications of the H+-efflux assay, and with the abilities of PfCRTEcu1110, PfCRT7G8 and PfCRTDd2 (but not PfCRT3D7) to transport CQ when tested under normal conditions in the oocyte system,. Unless labelled ns, P < 0.05 relative to the absence of a test solute. b Left: schematic showing the inhibition of PfCRT by verapamil (VP) or the quinine dimer Q2C in the H+-efflux assay. Right: the increase in the rate of DV alkalinisation caused by saquinavir (SQV; 10 μM) and Ac-YF-5-NH2 (10 μM) is inhibited by VP (50 μM) and Q2C (1 μM). The statistical analyses were performed relative to the SQV or Ac-YF-5-NH2 controls. c SQV (left) and Ac-YF-5-NH2 (right) increase the rate of DV alkalinisation in a concentration-dependent manner. d Concentration-dependence of the _trans_-stimulation of [3H]CQ transport via PfCRT by SQV (left) and Ac-YF-5-NH2 (right) in Xenopus oocytes. The non-expressing oocyte data overlays the data obtained with oocytes expressing PfNT1. The data are the mean of n = 5 independent experiments (each yielding similar results and overlaid as individual data points in a and b), and the error is the SEM. Where not visible, the error bars fall within the symbols. The asterisks denote a significant difference from the relevant C4Dd2 (red asterisks), C67G8 (orange asterisks) or C2GC03 (blue asterisks) control; *P < 0.05, **P < 0.01, ***P < 0.001, ns: non-significant (one-way ANOVA). The source datasets are provided as a Source Data file.
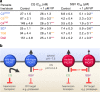
Fig. 6. CQ-resistance-conferring isoforms of PfCRT increase parasite susceptibility to saquinavir.
a The antiplasmodial activities of CQ and saquinavir (SQV) against CQ-sensitive (C2GC03 and 3D7) and CQ-resistant (C67G8, C4Dd2, 7G8 and Dd2) parasites in the presence or absence of verapamil (VP). The data are the mean of n = 5 independent experiments (each yielding similar results) and the error is the SEM. The asterisks denote a significant difference from the relevant control (red asterisks, C4Dd2 or Dd2 control; orange asterisks, C67G8 or 7G8 control; blue asterisks, C2GC03 or 3D7 control); **P < 0.01, ***P < 0.001, ns: non-significant (one-way ANOVA). The source datasets are provided as a Source Data file. b Mechanistic model for the increased susceptibility of CQ-resistant parasites to SQV. PfCRT3D7 (wild-type PfCRT) lacks detectable CQ transport activity, whereas the CQ-resistance-conferring isoforms of PfCRT (mutant PfCRT) efflux CQ from the DV. VP inhibits CQ transport via the mutant transporters, thereby re-sensitising the CQ-resistant parasites to this antimalarial drug. By contrast, PfCRT3D7 has a greater capacity for transporting SQV out of the DV than do the mutant isoforms. Moreover, it is the wild-type protein that confers reduced sensitivity to SQV. Given that VP (1) inhibits SQV transport via both the wild-type and mutant PfCRT transporters and (2) increases the antiplasmodial activity of SQV in all of the parasite types, our findings indicate that SQV acts on a target within the DV.
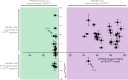
Fig. 7. The peptide substrates of PfCRT accumulate in parasites expressing mutant isoforms of the transporter.
Host-derived peptides in erythrocytes infected with C2GC03 or C4Dd2 parasites were quantified using tandem liquid-chromatography mass-spectrometry and the peptide levels within the C4Dd2 line were expressed relative to those measured in the C2GC03 parasites (Supplementary Data 3 and 5). For peptides containing 4–11 residues, a positive relationship exists between the ability to _trans_-stimulate CQ transport via PfCRT3D7 and accumulation within the CQ-resistant C4Dd2 parasites. The analysis used the PfCRT3D7_trans_-stimulation dataset, rather than that generated for PfCRTDd2, because the peptides most likely to accumulate in the C4Dd2 line will include those that are very poor substrates of (or no longer transported by) PfCRTDd2. An analysis of the data with a Bayesian Information Criteria model identified two distinct populations. The _trans_-stimulation data are the mean of four independent experiments (each yielding similar results) and the peptide accumulation data are the mean of 2–6 independent experiments (each yielding similar results). Error bars are shown for data points that are n ≥ 3; the Y error is the SD and the X error is the SEM. The source datasets are provided as a Source Data file.
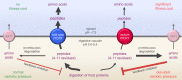
Fig. 8. Model for the natural function and physiological role of PfCRT.
PfCRT transports host-derived peptides 4–11 residues in length, and of varying composition and charge, out of the DV and into the parasite’s cytosol. Wild-type PfCRT transports a broader range of peptides, and has a higher capacity for peptide transport, than most CQ-resistance-conferring isoforms of PfCRT. The diminished abilities of the mutant transporters to efflux peptides results in the accumulation of these peptides within CQ-resistant parasites. Moreover, the promiscuous degradation of the accumulated peptides leads to the build-up of small peptide fragments and amino acids, which exerts further osmotic pressure upon the DV and causes feedback inhibition of the digestion of host proteins. PfCRT therefore serves two roles: (1) it prevents osmotic stress of the DV by exporting peptides and (2) it delivers these peptides to the cytosol where they are degraded into amino acids to fuel parasite growth. AAT1: amino acid transporter 1.
Similar articles
- Chloroquine transport via the malaria parasite's chloroquine resistance transporter.
Martin RE, Marchetti RV, Cowan AI, Howitt SM, Bröer S, Kirk K. Martin RE, et al. Science. 2009 Sep 25;325(5948):1680-2. doi: 10.1126/science.1175667. Science. 2009. PMID: 19779197 - A 2-amino quinoline, 5-(3-(2-(7-chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid, interacts with PfMDR1 and inhibits its drug transport in Plasmodium falciparum.
Edaye S, Reiling SJ, Leimanis ML, Wunderlich J, Rohrbach P, Georges E. Edaye S, et al. Mol Biochem Parasitol. 2014 Jun;195(1):34-42. doi: 10.1016/j.molbiopara.2014.05.006. Epub 2014 Jun 8. Mol Biochem Parasitol. 2014. PMID: 24914817 - Functional characteristics of the malaria parasite's "chloroquine resistance transporter": implications for chemotherapy.
Summers RL, Martin RE. Summers RL, et al. Virulence. 2010 Jul-Aug;1(4):304-8. doi: 10.4161/viru.1.4.12012. Virulence. 2010. PMID: 21178460 - PfCRT and its role in antimalarial drug resistance.
Ecker A, Lehane AM, Clain J, Fidock DA. Ecker A, et al. Trends Parasitol. 2012 Nov;28(11):504-14. doi: 10.1016/j.pt.2012.08.002. Epub 2012 Sep 25. Trends Parasitol. 2012. PMID: 23020971 Free PMC article. Review. - Is PfCRT a channel or a carrier? Two competing models explaining chloroquine resistance in Plasmodium falciparum.
Sanchez CP, Stein WD, Lanzer M. Sanchez CP, et al. Trends Parasitol. 2007 Jul;23(7):332-9. doi: 10.1016/j.pt.2007.04.013. Epub 2007 May 10. Trends Parasitol. 2007. PMID: 17493873 Review.
Cited by
- The Toxoplasma plant-like vacuolar compartment (PLVAC).
Stasic AJ, Moreno SNJ, Carruthers VB, Dou Z. Stasic AJ, et al. J Eukaryot Microbiol. 2022 Nov;69(6):e12951. doi: 10.1111/jeu.12951. Epub 2022 Oct 27. J Eukaryot Microbiol. 2022. PMID: 36218001 Free PMC article. Review. - Monitoring antimalarial drug-resistance markers in Somalia.
Jalei AA, Na-Bangchang K, Muhamad P, Chaijaroenkul W. Jalei AA, et al. Parasites Hosts Dis. 2023 Feb;61(1):78-83. doi: 10.3347/PHD.22140. Epub 2023 Feb 22. Parasites Hosts Dis. 2023. PMID: 37170467 Free PMC article. - The Digestive Vacuole of the Malaria Parasite: A Specialized Lysosome.
Wiser MF. Wiser MF. Pathogens. 2024 Feb 20;13(3):182. doi: 10.3390/pathogens13030182. Pathogens. 2024. PMID: 38535526 Free PMC article. Review. - Deletion of the chloroquine resistance transporter gene confers reduced piperaquine susceptibility to the rodent malaria parasite Plasmodium berghei.
Hirai M, Arai M, Hayamichi S, Uchida A, Sudo M, Kubota R, Shinzawa N, Mita T. Hirai M, et al. Antimicrob Agents Chemother. 2025 Apr 2;69(4):e0158924. doi: 10.1128/aac.01589-24. Epub 2025 Feb 24. Antimicrob Agents Chemother. 2025. PMID: 39992104 Free PMC article. - Additional PfCRT mutations driven by selective pressure for improved fitness can result in the loss of piperaquine resistance and altered Plasmodium falciparum physiology.
Hagenah LM, Dhingra SK, Small-Saunders JL, Qahash T, Willems A, Schindler KA, Rangel GW, Gil-Iturbe E, Kim J, Akhundova E, Yeo T, Okombo J, Mancia F, Quick M, Roepe PD, Llinás M, Fidock DA. Hagenah LM, et al. mBio. 2024 Jan 16;15(1):e0183223. doi: 10.1128/mbio.01832-23. Epub 2023 Dec 7. mBio. 2024. PMID: 38059639 Free PMC article.
References
- Hayward R, Saliba KJ, Kirk K. The pH of the digestive vacuole of Plasmodium falciparum is not associated with chloroquine resistance. J. Cell Sci. 2006;119:1016–1025. - PubMed
- Orjih AU, Ryerse JS, Fitch CD. Hemoglobin catabolism and the killing of intraerythrocytic Plasmodium falciparum by chloroquine. Experientia. 1994;50:34–39. - PubMed
- Becker K, et al. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int. J. Parasitol. 2004;34:163–189. - PubMed
- Rosenthal PJ. Falcipains and other cysteine proteases of malaria parasites. Adv. Exp. Med. Biol. 2011;712:30–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources