Voltage-gated potassium channel proteins and stereoselective S-nitroso-l-cysteine signaling - PubMed (original) (raw)
. 2020 Sep 17;5(18):e134174.
doi: 10.1172/jci.insight.134174.
Benjamin Gaston 1 2 3, Jürgen Bosch 2, James Seckler 2, Diana Kunze 4, Janna Kiselar 5, Nadzeya Marozkina 2, Craig A Hodges 2, Patrick Wintrobe 6, Kellen McGee 2, Tatiana S Morozkina 7, Spencer T Burton 2, Tristan Lewis 3, Timothy Strassmaier 8, Paulina Getsy 3, James N Bates 9, Stephen J Lewis 2 10
Affiliations
- PMID: 32790645
- PMCID: PMC7526540
- DOI: 10.1172/jci.insight.134174
Voltage-gated potassium channel proteins and stereoselective S-nitroso-l-cysteine signaling
Benjamin Gaston et al. JCI Insight. 2020.
Abstract
S-nitroso-l-cysteine (L-CSNO) behaves as a ligand. Its soluble guanylate cyclase-independent (sGC-independent) effects are stereoselective - that is, not recapitulated by S-nitroso-d-cysteine (D-CSNO) - and are inhibited by chemical congeners. However, candidate L-CSNO receptors have not been identified. Here, we have used 2 complementary affinity chromatography assays - followed by unbiased proteomic analysis - to identify voltage-gated K+ channel (Kv) proteins as binding partners for L-CSNO. Stereoselective L-CSNO-Kv interaction was confirmed structurally and functionally using surface plasmon resonance spectroscopy; hydrogen deuterium exchange; and, in Kv1.1/Kv1.2/Kvβ2-overexpressing cells, patch clamp assays. Remarkably, these sGC-independent L-CSNO effects did not involve S-nitrosylation of Kv proteins. In isolated rat and mouse respiratory control (petrosyl) ganglia, L-CSNO stereoselectively inhibited Kv channel function. Genetic ablation of Kv1.1 prevented this effect. In intact animals, L-CSNO injection at the level of the carotid body dramatically and stereoselectively increased minute ventilation while having no effect on blood pressure; this effect was inhibited by the L-CSNO congener S-methyl-l-cysteine. Kv proteins are physiologically relevant targets of endogenous L-CSNO. This may be a signaling pathway of broad relevance.
Keywords: Cell Biology; Respiration.
Conflict of interest statement
Conflict of interest: BG is a founder and equity owner in Lake Effect Pharma LLC. JNB and SJL are inventors on US patent 7,226,766 (ref. 20).
Figures
Figure 1. Identification of voltage-gated K+ channel proteins as binding partners for L-CSNO.
(A) Method 1. Murine cortex membrane proteins (50 μg/ lane; 2 sets of experiments using 1 C57BL/6 WT mouse) underwent native PAGE, then were incubated (30 minutes; dark; 27°C) with L-CSNO (50 μM) with or without 100 μM each L-CSMe and L-CSφ. Rinsed gels were developed with 40 μM DAF2 (30 minutes; dark; 27°C), and fluorescing bands (arrow) analyzed by LC-MS (compared with the control lane). Note that, because this is native PAGE, proteins were not separated before electrophoresis. (B) Method 2. Cysteine (100 mM) coupled to AminoLink Plus resin (4 hours, 27°C) or resin alone was incubated with EtONO (10 minutes; dark; 27°C). Pink color demonstrates _S_-nitrosothiol formation on the column (37). (C) Membrane proteins as in A (different animal) were loaded on columns (B) (30 min; dark; 27°C), washed, then eluted with Laemmli buffer followed by 0.1 M glycine, pH 3.5. Eluate underwent SDS-PAGE, and bands (including that shown by the arrow, 20 kDa) were analyzed by LC-MS in comparison with the control lane. Both methods identified several Kv channel proteins (see Supplemental Tables 2 and 3). Note that, because native PAGE was used (proteins were not separated before electrophoresis) in A, and a broad region of discordance was excised in C, multiple molecular weight proteins were evident by LC-MS. (D) Proteins as in A from WT and Kv1.1–/– mice (2 sets of experiments using WT and C57BL/6 background mice) were loaded on and eluted from columns as in B and C, and immunoblotted for Kv1.1 and Kvβ2.
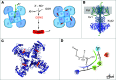
Figure 2. SyncroPatch analysis of the Shaker channel constructs.
(A) Kv1.2/Kvβ2 crystal structure (PDB 2A79). The homotetramer of Kv1.2 is in the brown ribbon; Kvβ2 is the green surface; the plasma membrane (PM) is gray. (B) Schematic drawing of the individual cell lines after transfection with Kv1.1, Kv1.2, and Kvβ2. Not all cells will express all proteins; this schematic shows all permutations, with the corresponding experimental patch clamp results. Icons surrounded by red boxes show no conductance, while icons with a light green background show specific K+ channel activity. Gray boxes indicate possible conformations that cannot be distinguished based on conductance. (C) Conductance characteristics of the different cells lines upon voltage gate clamping from –120 mV to +80 mV (n = 204 single-cell patch clamp studies, with 20 untransformed, 20 Kvβ2 alone, 26 Kv1.1 alone, 28 Kv1.2 alone, 28 Kv1.1/Kvβ2, 25 Kv1.2/Kvβ2, 27 Kv1.1/Kv1.2, and 61 triple-expressing). Current at each voltage above 20 mV is greater for the triple-overexpressing than for the other cells.) (D) Conductance of each cell line at +80 mV resting potential. Note that only Kv1.1/Kv1.2 and Kv1.1/Kv1.2/Kvβ2 show currents >500 pA. n is the same as described in C. (E) Examples of individual traces, showing 11 sweeps corresponding to the ramping protocol for different cells. Black is +80 mV. All traces are on the same scale. (F) Effect of L-SNOC on K+ current block in either the double or triple cell line. n = 8 cells in each condition; P = 0.002 by Mann-Whitney rank-sum test t test. (G) IC50 determination of L-CSNO on triple cell line with currents >500 pA represented as % block (error bars represent a confidence level of 95% [CL 95]) compared with TEA full block. There is no block with either vehicle (green) or D-CSNO (red). n = 8 in the L-CSNO group and n = 8 in the D-CSNO group. At concentrations greater than 500 nM (n = 236 points analyzed), inhibition by L-CSNO is greater than inhibition by D-CSNO (P = 0.0001 vs. D-CSNO and P = 0.0048 vs. vehicle, by Kruskal-Wallis test). Data are presented as median ± 95% CI.
Figure 3. L-CSNO binding to Kv proteins.
L-CSNO has unique binding interactions with the intracellular Kv proteins Kv1.1α T1 (A–E) and Kvβ2 (F) (see also Supplemental Figure 3). (A) The SPR binding response of L-CSNO, D-CSNO, or
l
-cysteine to Kv1.1α T1. The binding isotherm for each protein-ligand interaction is represented in the inset of each graph. n = 25 measurements each. Data are mean ± SEM.(B) CD analysis of Kv1.1α T1 in the presence of increasing amounts of L-CSNO and D-CSNO. n = 7 measurements each. (C) Secondary structure changes of Kv1.1α T1 upon titration with L-CSNO or D-CSNO. (D) Substrate stereoselectivity and thermal stability of Kv1.1α T1. Melting temperature increases by 20°C upon L-CSNO binding to Kv1.1α T1. km, Michaelis constant; ka, association constant; kd, dissociation constant; KD, affinity. (E) Differential deuterium uptake after a 15 minute pulse between the unliganded Kvβ and in solution with L-CSNO, D-CSNO,
l
-cysteine, or EtONO. Blue peptides represent a greater than 20% rigidification; green peptides represent between 0% and 20% rigidification. (F) Differential deuterium uptake after a 1 minute pulse between the unliganded Kv1.1α T1 and in solution with L-CSNO, D-CSNO, or
l
-cysteine. Blue peptides represent a greater than 20% rigidification. Green peptides represent between 0% and 20% rigidification, and gray peptides represent no significant rigidification. NADPH is shown in teal spheres.
Figure 4. Voltage-sensitive K+ currents are inhibited by L-CSNO and hypoxia.
(A). Extracellular L-CSNO reduced K+ current elicited by a slow voltage ramp from –80 to +60 mV in newborn rat petrosal ganglion cells studied by patch clamp. (B) After DTx, L-CSNO produced no further reduction in K+ current (n = 3 animals each). DTx blocks approximately 20% of the total K+ current (28). (C) D-CSNO had no effect on K+ current (n = 4, 100 μM to 1 mM). (D and E) As in rat neurons, L-CSNO inhibited DTx-sensitive voltage-gated K+ current relative to inhibition by subsequent DTx in WT murine ganglia in a dose-dependent fashion (D; n = 6; Inhmax = 0.4), but (E) maximal doses (>20 μM) had no effect in Kv1.1–/– murine neurons (n = 4 Kv WT neurons and n = 6 Kv1.1–/– neurons; P < 0.05 by ANOVA).
Figure 5. Stereoselective biological activity of the endogenous ligand, L-CSNO.
(A) Maximal increases in VE elicited by arterial injections of L-CSNO and D-CSNO in conscious Sprague-Dawley rats (n = 9). *P < 0.05, by ANOVA on ranks; †P < 0.05, D-CSNO versus L-CSNO. Mean ± SEM. IA, intra-arterial. (B) Changes in frequency of breathing (Freq), TV, VE [minute ventilation, MV]), and MAP elicited by a 25 nmol/kg dose of L-CSNO (n = 9 each; mean ± SEM at each time point). (C) Maximal increases in VE elicited by L-CSNO in Sprague-Dawley rats receiving infusions of vehicle (VEH; 0.1% DMSO in saline, 20 μL/min), ODQ (2 mg/kg bolus followed by 50 μg/kg/min, i.v.), or S-methyl-L-cysteine (SMC or L-CSMe) (10 μmol/kg/min, i.v.). n = 8–10 animals/experiment. *P < 0.05, SMC versus VEH or ODQ. Mean ± SEM. (D) Mice preinstrumented with arterial catheters at the level of the CB were treated with the L-CSNO congener L-CSMe (filled circles) or vehicle (open circles), then exposed sequentially to 10% oxygen or room air. L-SMC almost completely ablated the normal hypoxic response and recovery (n = 9 each; P < 0.001). At each time point, mean ± SEM. (E) The effect of L-CSNO is mediated by the CB. Mice underwent CSN (CSNO transection and carotid artery cannulation (as in Figure 5D). After a 3 week recovery, they were exposed to infusions of L-CSNO. Mice with intact CSN (SHAM, open circles) had a normal response to carotid L-CSNO infusion, while those with CSN transection (closed circles) did not (n = 12 each; mean ± SEM; P < 0.001 by ANOVA on ranks). (F) Detection of L-CSNO in blood samples. n = 3 each; median and CI. *P < 0.05, significant value; †P < 0.05, venous versus arterial, by ANOVA on ranks.
Figure 6. L-CSNO inhibition of voltage-gated K+ current.
(A) Top view: Schematic showing L-CSNO’s effect of altering the structure of the Kv multimer. The HDX data (Figure 3 and Supplemental Figure 3) show that the tertiary structure of Kvβ2 is altered by L-CSNO binding to make the protein more concave, particularly at the base near the site of its interaction with Kvα proteins. Kv1.1 T1 is also affected (Figure 3 and Supplemental Figure 3), but patch clamp data suggest that the interaction with Kvβ2 is essential for a functional effect (Figure 2). L-CSNO is formed from GSNO by γ-glutamyl transpeptidase and downstream dipeptidases (1), signaling increased VE (1). GSNO is formed, in turn, by NOS activation, oxyhemoglobin desaturation, ceruloplasmin and other metalloproteins capable of transferring NO+ equivalents to thiolate anions. Uniquely, NO is not transferred to the Kv proteins. D-CSNO does not share the activity of L-CSNO (Figures 2–5), though both isomers form NO at the same rate (20). (B) Lateral view of the Kvα/Kvβ complex in the plasma membrane, as in Figure 2A: side view of K+ exit from the cell. (C) Based on HDX, approximate sites of L-SNO binding in the 4 Kvβ2 subunits. (D) In silico estimates of L-CSNO interaction with Kvβ2 amino acids using ChemDraw and Schrödinger Maestro software. Reproduced with permission from V. Ferrante.
Similar articles
- S-Nitroso-l-cysteine and ventilatory drive: A pediatric perspective.
Hubbard D, Tutrow K, Gaston B. Hubbard D, et al. Pediatr Pulmonol. 2022 Oct;57(10):2291-2297. doi: 10.1002/ppul.26036. Epub 2022 Jul 24. Pediatr Pulmonol. 2022. PMID: 35785452 Free PMC article. Review. - Identification of stereoselective transporters for S-nitroso-L-cysteine: role of LAT1 and LAT2 in biological activity of S-nitrosothiols.
Li S, Whorton AR. Li S, et al. J Biol Chem. 2005 May 20;280(20):20102-10. doi: 10.1074/jbc.M413164200. Epub 2005 Mar 15. J Biol Chem. 2005. PMID: 15769744 - RhoA inactivation by S-nitrosylation regulates vascular smooth muscle contractive signaling.
Lin L, Xu C, Carraway MS, Piantadosi CA, Whorton AR, Li S. Lin L, et al. Nitric Oxide. 2018 Apr 1;74:56-64. doi: 10.1016/j.niox.2018.01.007. Epub 2018 Jan 31. Nitric Oxide. 2018. PMID: 29355776 - S-Nitroso-L-Cysteine Ameliorated Pulmonary Hypertension in the MCT-Induced Rats through Anti-ROS and Anti-Inflammatory Pathways.
Wang M, Luo P, Shi W, Guo J, Huo S, Yan D, Peng L, Zhang C, Lv J, Lin L, Li S. Wang M, et al. Oxid Med Cell Longev. 2021 Jan 28;2021:6621232. doi: 10.1155/2021/6621232. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33574976 Free PMC article. Retracted. - Synergistic inhibition of the maximum conductance of Kv1.5 channels by extracellular K+ reduction and acidification.
Fedida D, Zhang S, Kwan DC, Eduljee C, Kehl SJ. Fedida D, et al. Cell Biochem Biophys. 2005;43(2):231-42. doi: 10.1385/CBB:43:2:231. Cell Biochem Biophys. 2005. PMID: 16049348 Review.
Cited by
- Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via _β_Cys93.
Hausladen A, Qian Z, Zhang R, Premont RT, Stamler JS. Hausladen A, et al. J Pharmacol Exp Ther. 2022 Jul;382(1):1-10. doi: 10.1124/jpet.122.001194. Epub 2022 May 5. J Pharmacol Exp Ther. 2022. PMID: 35512801 Free PMC article. - The cell-permeant antioxidant D-thiol ester D-cysteine ethyl ester overcomes physical dependence to morphine in male Sprague Dawley rats.
Getsy PM, Coffee GA, Bates JN, Parran T, Hoffer L, Baby SM, MacFarlane PM, Knauss ZT, Damron DS, Hsieh YH, Bubier JA, Mueller D, Lewis SJ. Getsy PM, et al. Front Pharmacol. 2024 Aug 26;15:1444574. doi: 10.3389/fphar.2024.1444574. eCollection 2024. Front Pharmacol. 2024. PMID: 39253377 Free PMC article. - L-cysteine ethylester reverses the adverse effects of morphine on breathing and arterial blood-gas chemistry while minimally affecting antinociception in unanesthetized rats.
Baby SM, May WJ, Young AP, Wilson CG, Getsy PM, Coffee GA, Lewis THJ, Hsieh YH, Bates JN, Lewis SJ. Baby SM, et al. Biomed Pharmacother. 2024 Feb;171:116081. doi: 10.1016/j.biopha.2023.116081. Epub 2024 Jan 13. Biomed Pharmacother. 2024. PMID: 38219385 Free PMC article. - Hypoxia releases S-nitrosocysteine from carotid body glomus cells-relevance to expression of the hypoxic ventilatory response.
Seckler JM, Getsy PM, May WJ, Gaston B, Baby SM, Lewis THJ, Bates JN, Lewis SJ. Seckler JM, et al. Front Pharmacol. 2023 Oct 11;14:1250154. doi: 10.3389/fphar.2023.1250154. eCollection 2023. Front Pharmacol. 2023. PMID: 37886129 Free PMC article. - Nitrosyl factors play a vital role in the ventilatory depressant effects of fentanyl in unanesthetized rats.
Seckler JM, Grossfield A, May WJ, Getsy PM, Lewis SJ. Seckler JM, et al. Biomed Pharmacother. 2022 Feb;146:112571. doi: 10.1016/j.biopha.2021.112571. Epub 2021 Dec 22. Biomed Pharmacother. 2022. PMID: 34953397 Free PMC article.
References
- Lipton AJ, et al. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413(6852):171–174. - PubMed
- Kluge I, Gutteck-Amsler U, Zollinger M, Do KQ. S-nitrosoglutathione in rat cerebellum: identification and quantification by liquid chromatography-mass spectrometry. J Neurochem. 1997;69(6):2599–2607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL125245/HL/NHLBI NIH HHS/United States
- S10 OD026882/OD/NIH HHS/United States
- P01 HL101871/HL/NHLBI NIH HHS/United States
- P01 HL128192/HL/NHLBI NIH HHS/United States
- U01 DA051373/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources