Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis - PubMed (original) (raw)
Review
Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis
S DiNardo et al. Nature. 1988.
Abstract
A regulatory cascade, initiated during the syncytial stage of embryogenesis, culminates in the striped pattern of engrailed gene expression at the cellular blastoderm stage. The early regulatory genes, for example the pair-rule genes, are expressed transiently and as their products decay a distinct regulatory programme involving segment polarity genes takes over. This late programme maintains and perhaps modifies the striped pattern of engrailed expression through interactions that may involve cell communication.
Figures
Fig. 1
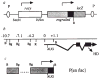
Construction of an en-lacZ fusion gene (not to scale), a, schematic diagram of P(en/lac) indicating sites for P-mediated integration (P); the rosy gene (open box); upstream and non-coding (stippled) and coding (filled) regions from engrailed; and lacZ coding sequences (hatched). An SV40 polyadenylation signal is at the end of lacZ. b, Map of en genomic region showing particular restriction site landmarks, the major transcription unit with start site (0) and presumed translation start site (AUG) and the portions of exons two and three (solid boxes) encoding the homoeodomain (HD),,. c, Specific genomic en regions used in constructing P(en/lac) (aligned with b). Dots represent the deletion of sequences between coordinates −4.2 and −7.1. Restriction sites: B, _Bam_HI; Bg, _Bgl_II; H, _Hin_dIII; R, _Eco_RI; Sm, _Sma_I; symbol (/), joined by blunt-end ligation. Arrows indicate the direction of transcription. Methods. Transgenic lines were established using ‘wings-clipped’ helper, and an _Sgs_–4Ber–-1, _cn; ry_506 host. Lines were established from three independent G0 adults; the insertion mapped to chromosome II in one line and to chromosome III in the other two.
Fig. 2
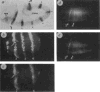
Antibody-staining of P(en/lac) transgenic embryos show that regulatory sequences controlling establishment of even-numbered en stripes are functionally distinct from those controlling establishment of odd-numbered stripes. Anterior left and ventral down in all panels, unless indicated otherwise, a, P(en/lac) transformant stained with rabbit anti-_β_-galactosidase antibody to visualize the expression from P(en/lac), 3.5–4h after egg laying (AEL). Seven ectodermal stripes are evident (indicated by dots), corresponding to en stripes 2, 4, 6, 8, 10, 12 and 14, which mark the posterior portions of the maxillary, first and third thoracic, and second, fourth, sixth and eighth abdominal primordia. Variable expression seen in the anterior (amg) and posterior (pmg) midgut primordia is not part of the normal en expression program. This expression may be due to sequences from en, or may be spurious, since expression from similar vectors in mesodermal and gut primordia has been reported. b, c: Magnified (ventral) view of a doubly-labelled embryo (mouse anti -en and rabbit anti-_β_-gal) reveals expression of endogenous en (b) and of P(en/lac) (c), 4.5 h AEL. The stripes of en expression are 1(Mn), 2(Max), 3(La) and 4(T1). Expression of _β_-gal is coincident with even-numbered en stripes 2(Max) and 4(T1); arrows indicate the same cells in b and c. There is no detectable _β_-gal expression at positions corresponding to odd-numbered en stripes. In even-numbered stripes there is virtually one-to-one correspondence between _β_-gal and en expressing cells. The occasional cell containing low levels of _β_-gal antigen but no detectable en is indicated (open arrow). The _β_-gal signal is not as discrete as the en signal since the en-_β_-gal fusion protein is not restricted to the nucleus, d, e: Embryos were doubly-labelled for en expression (d) and _β_-gal expression (e) at the onset of gastrulation, 3 h AEL. Expression from P(en/lac) is induced at about the same developmental stage as en, although there may be a slight lag in induction or accumulation. Arrows point to co-expression in 2(Max), and in 4(T1), where _β_-gal is just detectable. Methods. Preparation of embryos for immunocytochemistry was as in refs , . Rabbit anti-_β_-gal was from Cappel, rabbit anti-en, (ref. 10); mouse monoclonal anti-en (and inv), a gift of K. Coleman, C. Goodman and T. Kornberg. Final magnification was ×130, except for b and c which were ×520.
Fig. 3
P(en/lac) uncovers a distinct late program of control over en, which is regulated by segment polarity genes, a, staining for _β_-gal, 5 h AEL, anterior left and ventral down (×130). The seven _β_-gal stripes (dots) are beginning to fade. Note the spotty appearance laterally (open arrows). As development proceeds this signal fades ventrally. In addition, note that some cells at dorso-lateral positions are beginning to accumulate _β_-gal signal (solid arrows). The signal in the dorsal ectoderm will be heavily induced during subsequent stages. Though restricted to dorsal regions, the position of this signal corresponds accurately to even-and odd-numbered en stripes, b, Staining for _β_-gal at the onset of germband retraction, 7–8 h AEL, ventro-lateral view (×130). Expression of P(en/lac) in ventral areas is low, but expression at dorso-lateral positions is quite high. Expression in both ventral and dorsal positions occurs in the posterior portions of 11 segment primordia: Labial, T1–T3, and A1–A7. Significant expression of P(en/lac) in Mandibular and Maxillary primordia and the terminalia during this late programme is usually not observed. Expression in dorsal-most A8 is sometimes observed. Bracketed area is magnified (from a different embryo) in c and d. VM, ventral midline. c, d: Magnified view (×520) of a doubly-labelled embryo at the turn of the germband (A2, A3 and A4 region) showing one to one correspondence between en (c) and _β_-gal (d) expressing cells, e, f: Same as c, d but in ptc mutant background, with arrow showing that P(en/lac) is ectopically induced (f) as is the endogenous en gene (e).
Fig. 4
Premature decay of en expression in wg and hh mutants. Embryos, anterior left and ventral down, were stained with anti-en-antibody, a, Mutant _wg_cx4: 4–5 h AEL(×130). Most en stripes are faint, but still visible above background. The Max and La stripes are affected least. Similar results have been obtained in wg_L5, wg_IG22, and also in _wg_IL114ts when aged at non-permissive temperatures (25–28 °C). In wild-type embryos en antigen can be visualized in cells until the onset of cuticular differentiation, 12–14h AEL. b, Mutant _wg_cx4: magnified (×520) view (in a different embryo) of bracketed region shown in (a) as signal from en expression decays. Particular en stripes lose signal at varying times; thus, these two adjacent en stripes clearly show that the decay in en expression occurs while cells are still part of the ectodermal cell sheet (arrowhead, decaying signal), c, Mutant _wg_cx4: 6.5 h AEL (×130). The remaining en signal is lost from ectodermal cells, except in Max and La regions. The blurred ventral signal is due to en expression in cells (out of this focal plane) of the developing central nervous system, _d, hh_6N: 6 h AEL (×130). Note the spotty and thin appearance of en stripes. Similar results are found for _arm_xk22, _fu_113, _fu_113/_fu_94, hh_6n/hh_9k, and in _wg_IL114ts (raised at 16–18 °C). The segment polarity mutants gooseberry and _cubitus interrupts_D have not been studied.
Fig. 5
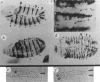
Reprogramming of cells to express en in patched mutants. Mutant embryos _ptc_IN108were stained with anti-en-antibody, a, Germband extended embryo (4.5 h AEL), anterior left and ventral down (×130). The normal 14 en stripes, (established at the cellular blastoderm stage) are now two to three cells wide and are indicated by dots. The patches and stripe of _en_-expressing cells anterior to stripe 1 resemble wild-type. Cells newly accumulating en antigen are indicated (arrowheads). The stage at which ectopic expression is first detectable cannot be due to leakiness of the ptc allele because the same delay is observed in _ptc_IN108 homozygotes as in the deficiency/_ptc_IN108 heteroallelic combination. A furrow in the ectodermal cell sheet is becoming visible between each pair of en stripes at the approximate position of ectopic induction (open arrows). The asterisk marks a cell group that induces en at this stage in wild-type also, b, Ventral view, 6 h AEL (×130). Eight en stripes are visible. More cells now express en between each pair of normal stripes, giving the appearance of an eccentrically positioned, incomplete stripe (arrowheads), c, Magnified (×520) ventro-lateral view (6h AEL) showing three normal en stripes (white arrowheads) and cells that have induced en, located near and often within the deep furrows, d, Germband retracted (9 h AEL) anterior left ventral view (×130). A few normal stripes (dots) and the intervening ectopic stripes are indicated (arrowheads). A given ectopic stripe has a wavy appearance, fusing along part of its length alternately with a posteriorly located en stripe, and then ventro-laterally with an anteriorly located stripe (open arrows). This fusion may be the result of shifts in cell position during germband retraction. Such fusions and reorganization of en stripes are frequently seen at late developmental stages in many of the segmentation mutants, e, Wild-type fourth abdominal denticle belt with border (SB) between third (upper) and fourth (lower) segment indicated. f, Fourth abdominal denticle belt in mutant _ptc_1N108/Df(2R)ptc showing duplication of segment border (SB), and anterior row of denticles (open arrows). In _ptc_6p43, _en_IM99 double mutants (raised at 18–20 °C) both normal and duplicate anterior row denticles do not appear, showing dependence on en function. Methods. Stocks were obtained from C. Nusslein-Volhard and E. Wieschaus, from N. Baker (_wg_cx4), and J. Hooper and M. Scott (Df(2R)_ptc_44C–E). Crosses were performed in standard fashion, at 25 °C on molasses-cornmeal-agar. Embryos were prepared for immunocytochemical detection of en protein by affinity-purified rabbit anti-en antibody, Cuticles were prepared as in ref. .
Fig. 6
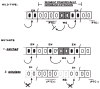
Interactions regulating en during post-blastoderm development. Wild-type: The segment primordium at germband extension stage (anterior to left) is represented as eight cells wide—the roughly four-cell wide primordia having expanded due to a post-cellular blastoderm cell division and some interdigitation of cells. Several domains within each primordium appear to exist, each ‘marked’ by the expression of, or by the requirement for the activity of, particular genes. Cells expressing en and wg account for two of the domains: the en domain comprises the last two cells of the primordium– (blackened nuclei); the anteriorly adjacent two cells constitute the wg domain (hatching). The analysis of ptc mutations suggests that the remaining cells of the primordium are divided into at least two domains: those that ectopically induce en in the absence of ptc activity, and those that do not. The placement of the _ptc_-requiring domain is based on the position of ectopic en expression in ptc mutants. It has been proposed that the activity of another gene, naked, is required for the anterior-most cells of the primordium. Each of the domains is presumably established at the cellular blastoderm stage by the action of pair-rule segmentation genes, as has been shown for en, and wg domains. The establishment of en expression constitutes the early regulatory programme of en. The second regulatory programme acts during post-blastoderm development as _wg_-expressing cells induce (or maintain) en expression in posteriorly located cells (arrow). Data presented here do not rule out other interactions. Mutants: (1) In the absence of ptc activity, en is induced in cells anterior to the wg domain. The induction requires wg (arrow to anterior), as no induction is detected in the absence of wg activity. Thus, in wild-type, wg induces en posterior to the wg domain, but is inhibited, directly or indirectly, from doing so anteriorly by ptc activity (blocking arrow shown in ‘wild-type’ panel). A cell (marked ‘?’) lies between the wg domain and the cell ectopically inducing en (see text). (2) the wg mutation leads to the premature loss of en expression (stippled nuclei), as a result of the loss of a morpho-genetic signal from wg expressing cells, perhaps the wg product itself. The hh, fu and/or arm products may play some role in the transduction of this signal. Cuticle pattern normally produced by en and _wg_-dependent cell types is missing, either due to cell death or transformation of fate (dashed cell outlines). It is not known if the wg mutation affects cells located anteriorly to the wg domain.
Similar articles
- Establishment and refinement of segmental pattern in the Drosophila embryo: spatial control of engrailed expression by pair-rule genes.
DiNardo S, O'Farrell PH. DiNardo S, et al. Genes Dev. 1987 Dec;1(10):1212-25. doi: 10.1101/gad.1.10.1212. Genes Dev. 1987. PMID: 3123316 - Changing role of even-skipped during the evolution of insect pattern formation.
Patel NH, Ball EE, Goodman CS. Patel NH, et al. Nature. 1992 May 28;357(6376):339-42. doi: 10.1038/357339a0. Nature. 1992. PMID: 1350328 - The initiation of pair-rule stripes in the Drosophila blastoderm.
Small S, Levine M. Small S, et al. Curr Opin Genet Dev. 1991 Aug;1(2):255-60. doi: 10.1016/s0959-437x(05)80079-6. Curr Opin Genet Dev. 1991. PMID: 1822273 Review. - Segment polarity genes and cell patterning within the Drosophila body segment.
Ingham PW. Ingham PW. Curr Opin Genet Dev. 1991 Aug;1(2):261-7. doi: 10.1016/s0959-437x(05)80080-2. Curr Opin Genet Dev. 1991. PMID: 1688007 Review.
Cited by
- Specification of the endocrine primordia controlling insect moulting and metamorphosis by the JAK/STAT signalling pathway.
García-Ferrés M, Sánchez-Higueras C, Espinosa-Vázquez JM, C-G Hombría J. García-Ferrés M, et al. PLoS Genet. 2022 Oct 3;18(10):e1010427. doi: 10.1371/journal.pgen.1010427. eCollection 2022 Oct. PLoS Genet. 2022. PMID: 36191039 Free PMC article. - Different temporal requirements for tartan and wingless in the formation of contractile interfaces at compartmental boundaries.
Sharrock TE, Evans J, Blanchard GB, Sanson B. Sharrock TE, et al. Development. 2022 Nov 1;149(21):dev200292. doi: 10.1242/dev.200292. Epub 2022 Oct 31. Development. 2022. PMID: 36178136 Free PMC article. - Extensive loss of Wnt genes in Tardigrada.
Chavarria RA, Game M, Arbelaez B, Ramnarine C, Snow ZK, Smith FW. Chavarria RA, et al. BMC Ecol Evol. 2021 Dec 27;21(1):223. doi: 10.1186/s12862-021-01954-y. BMC Ecol Evol. 2021. PMID: 34961481 Free PMC article. - The Emerging Mechanisms of Wnt Secretion and Signaling in Development.
Mehta S, Hingole S, Chaudhary V. Mehta S, et al. Front Cell Dev Biol. 2021 Aug 16;9:714746. doi: 10.3389/fcell.2021.714746. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34485301 Free PMC article. Review. - _Oncopeltus_-like gene expression patterns in Murgantia histrionica, a new hemipteran model system, suggest ancient regulatory network divergence.
Hernandez J, Pick L, Reding K. Hernandez J, et al. Evodevo. 2020 Apr 22;11:9. doi: 10.1186/s13227-020-00154-x. eCollection 2020. Evodevo. 2020. PMID: 32337018 Free PMC article.
References
- Kornberg T, et al. Cell. 1985;40:45–53. - PubMed
- Fjose A, McGinnis WJ, Gehring WJ. Nature. 1985;313:284–289. - PubMed
- Nüsslein-Volhard C, Wieschaus E. Nature. 1980;287:795–801. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases