Glucose transporters in the small intestine in health and disease - PubMed (original) (raw)
Review
Glucose transporters in the small intestine in health and disease
Hermann Koepsell. Pflugers Arch. 2020 Sep.
Abstract
Absorption of monosaccharides is mainly mediated by Na+-D-glucose cotransporter SGLT1 and the facititative transporters GLUT2 and GLUT5. SGLT1 and GLUT2 are relevant for absorption of D-glucose and D-galactose while GLUT5 is relevant for D-fructose absorption. SGLT1 and GLUT5 are constantly localized in the brush border membrane (BBM) of enterocytes, whereas GLUT2 is localized in the basolateral membrane (BLM) or the BBM plus BLM at low and high luminal D-glucose concentrations, respectively. At high luminal D-glucose, the abundance SGLT1 in the BBM is increased. Hence, D-glucose absorption at low luminal glucose is mediated via SGLT1 in the BBM and GLUT2 in the BLM whereas high-capacity D-glucose absorption at high luminal glucose is mediated by SGLT1 plus GLUT2 in the BBM and GLUT2 in the BLM. The review describes functions and regulations of SGLT1, GLUT2, and GLUT5 in the small intestine including diurnal variations and carbohydrate-dependent regulations. Also, the roles of SGLT1 and GLUT2 for secretion of enterohormones are discussed. Furthermore, diseases are described that are caused by malfunctions of small intestinal monosaccharide transporters, such as glucose-galactose malabsorption, Fanconi syndrome, and fructose intolerance. Moreover, it is reported how diabetes, small intestinal inflammation, parental nutrition, bariatric surgery, and metformin treatment affect expression of monosaccharide transporters in the small intestine. Finally, food components that decrease D-glucose absorption and drugs in development that inhibit or downregulate SGLT1 in the small intestine are compiled. Models for regulations and combined functions of glucose transporters, and for interplay between D-fructose transport and metabolism, are discussed.
Keywords: Bariatric surgery; Diabetes; Fructose intolerance; GLUT2; GLUT5; Glucose transporter; Glucose-galactose malabsorption; Regulation; SGLT1; Small intestine.
Figures
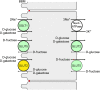
Fig. 1
Location of monosaccharide transporters in enterocytes that are involved in small intestinal absorption of
d
-glucose,
d
-galactose, and
d
-fructose. The locations were determined in different species including humans. Highly expressed transporters are outlined bold. Locations of monosaccharide transporters observed under various physiological and pathophysiological conditions are indicated in green. GLUT2 that was only observed in the BBM at high small intestinal
d
-glucose concentrations or in some pathological conditions is indicated in yellow. The Na++K+-ATPase in the BLM generating the inwardly directed Na+ gradient is also depicted
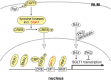
Fig. 2
Transcriptional regulation of SGLT1/Sglt1. Response elements in the promotor of human SGLT1 and components that were shown to be involved in transcriptional regulation of SGLT1/Sglt1 are indicated. Components that participate in transcriptional regulation in the small intestine are marked with yellow. If they participate in
d
-glucose-dependent regulation, they are indicated in red. EGFR, epithelial growth factor receptor; Rg1, ginsenoside component Rg1; SGK, serum- and corticoid-stimulated kinase; CREB, cAMP response element- binding protein- ; CBP, cAMP response element protein–binding protein; PER1, period circadian regulator 1; BMAL1, brain and muscle ANRT-like 1; SP1-1, specificity protein 1 subtype 1; HNF1, hepatic nuclear factor 1
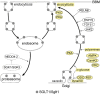
Fig. 3
Membrane trafficking of SGLT1/Sglt1. The scheme is based on experiments in which SGLT1 from human or rabbit was expressed in oocytes, and on experiments with mouse small intestine. Components which have been shown to be involved in short-term regulation of SGLT1/Sglt1 in the small intestine are indicated in yellow. The proteasomal degradation pathway is not well explored. RELM, resistin-like molecule; HSP, heat shock protein; TGF, tissue growth factor; JAK, Janus-activated kinase; AMPK, AMP-activated protein kinase; CamK, calmodulin-stimulated kinase; ODC, ornithine decarboxylase; NEDD, neural precursor cell expressed developmentally downregulated; SGK, serum- and glucocorticoid-stimulated kinase
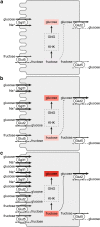
Fig. 4
Model depicting the presumed role of RS1 in
d
-glucose-dependent release of vesicles containing human SGLT1 from the Golgi (a, b) and the action of modified peptides derived from RS1-Reg (c) that downregulate SGLT1 in the BBM. RS1-Reg in RS1 is indicated in gray. TGN, _trans_-Golgi network; ODC, ornithine decarboxylase; BP, putrescine-binding protein of a budding protein complex that induces the release of vesicles containing human SGLT1 from the TGN
Fig. 5
Transcriptional regulation of GLUT2. Response elements in the promotor of human GLUT2 and transcription factors that are supposed to be involved in the regulation of GLUT2/Glut2 in the small intestine are indicated. SREBP-1c participates in
d
-glucose-dependent regulation of Glut2. C-EBP, CCAAT enhancer–binding protein; PPAR, peroxisome proliferator-activated receptor; RXR, retinoid X receptor; SREBP, sterol receptor element–binding protein; HNF, hepatic nuclear factor; FOX, forkhead box; p300, histone acetyltransferase p300
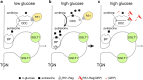
Fig. 6
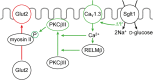
Model depicting components that are involved in targeting of Glut2 to the BBM at high luminal glucose concentrations. The underlying experiments were performed in rats. ∆Ψ, membrane depolarization due to Na+-
d
-glucose cotransport; Cav, voltage-dependent Ca2+channel; RELM, resistin-like molecule
Fig. 7
Plasma membrane localization and abundance of Sglt1, Glut2, and Glut3 in response to carbohydrates in the diet after ingestion of a carbohydrate-poor or a carbohydrate- and sucrose-rich meal. The underlying experiments were performed in rats. a Carbohydrate-poor diet after a carbohydrate-poor meal. b Carbohydrate-poor diet after a sucrose-rich meal. c Carbohydrate-rich diet after a sucrose-rich meal. KHK, ketohexokinase; GNG, gluconeogenesis
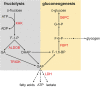
Fig. 8
Fructolysis and gluconeogenesis, and effects of removal of carbohydrate-responsive element (ChoRE)–binding protein in mice on fructose-dependent expression of the involved enzymes. Enzymes that are upregulated by high-fructose diet in the presence but not in the absence of ChoRE are indicated in red. Of note, also Glut5 in the luminal membrane of enterocytes mediating
d
-fructose uptake is only upregulated by high-fructose diet if ChoRE-binding protein is expressed in the enterocytes. Ketohexokinase (KHK), aldolase B (ALDOB), triosekinase (TRIOK), glucose-6-phosphatase (G6PC), fructose-1,6-biphosphatase (FBP1), and lactate dehydrogenase (LDH) are upregulated
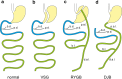
Fig. 9
Schematic representation of the most common bariatric surgery procedures. a Normal situation. b VSG, vertical sleeve gastrectomy. c RYGB, Roux-en-Y gastric bypass. d DJB, duodeno-jejunal bypass. Stomach yellow, duodenum blue, jejunum green. b.d, bile duct; p.d., pancreatic duct; p.a.l., proximal alimentary limb; d.a.l., distal alimentary limb; b.l., bile limb
Similar articles
- Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion.
Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, Veyhl-Wichmann M, Srinivasan A, Balen D, Breljak D, Rexhepaj R, Parker HE, Gribble FM, Reimann F, Lang F, Wiese S, Sabolic I, Sendtner M, Koepsell H. Gorboulev V, et al. Diabetes. 2012 Jan;61(1):187-96. doi: 10.2337/db11-1029. Epub 2011 Nov 28. Diabetes. 2012. PMID: 22124465 Free PMC article. - Diet effects on glucose absorption in the small intestine of neonatal calves: importance of intestinal mucosal growth, lactase activity, and glucose transporters.
Steinhoff-Wagner J, Zitnan R, Schönhusen U, Pfannkuche H, Hudakova M, Metges CC, Hammon HM. Steinhoff-Wagner J, et al. J Dairy Sci. 2014 Oct;97(10):6358-69. doi: 10.3168/jds.2014-8391. Epub 2014 Aug 6. J Dairy Sci. 2014. PMID: 25108868 - Expression of Na+/glucose co-transporter 1 (SGLT1) in the intestine of piglets weaned to different concentrations of dietary carbohydrate.
Moran AW, Al-Rammahi MA, Arora DK, Batchelor DJ, Coulter EA, Ionescu C, Bravo D, Shirazi-Beechey SP. Moran AW, et al. Br J Nutr. 2010 Sep;104(5):647-55. doi: 10.1017/S0007114510000954. Epub 2010 Apr 13. Br J Nutr. 2010. PMID: 20385036 - Intestinal absorption in health and disease--sugars.
Wright EM, Martín MG, Turk E. Wright EM, et al. Best Pract Res Clin Gastroenterol. 2003 Dec;17(6):943-56. doi: 10.1016/s1521-6918(03)00107-0. Best Pract Res Clin Gastroenterol. 2003. PMID: 14642859 Review. - Adaptation of intestinal nutrient transport in health and disease. Part I.
Thomson AB, Wild G. Thomson AB, et al. Dig Dis Sci. 1997 Mar;42(3):453-69. doi: 10.1023/a:1018807120691. Dig Dis Sci. 1997. PMID: 9073126 Review.
Cited by
- Role of milk carbohydrates in intestinal health of nursery pigs: a review.
Jang KB, Kim SW. Jang KB, et al. J Anim Sci Biotechnol. 2022 Jan 5;13(1):6. doi: 10.1186/s40104-021-00650-7. J Anim Sci Biotechnol. 2022. PMID: 34983676 Free PMC article. Review. - Importance of genetic sequencing studies in managing chronic neonatal diarrhea: a case report of a novel variant in the glucose-galactose transporter SLC5A1.
López-Mejía L, Guillén-Lopez S, Vela-Amieva M, Santillán-Martínez R, Abreu M, González-Herrra MD, Díaz-Martínez R, Reyes-Magaña JG. López-Mejía L, et al. Front Pediatr. 2024 Feb 19;12:1284671. doi: 10.3389/fped.2024.1284671. eCollection 2024. Front Pediatr. 2024. PMID: 38440183 Free PMC article. - Gut-brain communication and obesity: understanding functions of the vagus nerve.
Berthoud HR, Albaugh VL, Neuhuber WL. Berthoud HR, et al. J Clin Invest. 2021 May 17;131(10):e143770. doi: 10.1172/JCI143770. J Clin Invest. 2021. PMID: 33998597 Free PMC article. Review. - How dietary amino acids and high protein diets influence insulin secretion.
Yanagisawa Y. Yanagisawa Y. Physiol Rep. 2023 Jan;11(2):e15577. doi: 10.14814/phy2.15577. Physiol Rep. 2023. PMID: 36695783 Free PMC article. Review.
References
- Abdullah AM, Abdullah MA, Abdurrahman MB, al Husain MA (1992) Glucose-galactose malabsorption with renal stones in a Saudi child. Ann Trop Paediatr 12:327–329. 10.1080/02724936.1992.11747593 - PubMed
- Abdullah AMA, el-Mouzan MI, Shiekh OKE, Mazyad AA (1996) Congenital glucose-galactose malabsorption in Arab children. J Pediatr Gastroenterol Nutr 23:561–564. 10.1097/00005176-199612000-00008 - PubMed
- Abdul-Wahed A, Guilmeau S, Postic C (2017) Sweet sixteenth for ChREBP: established roles and future goals. Cell Metab 26:324–341. 10.1016/j.cmet.2017.07.004 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources