Identification of Uncharacterized Components of Prokaryotic Immune Systems and Their Diverse Eukaryotic Reformulations - PubMed (original) (raw)
. 2020 Nov 19;202(24):e00365-20.
doi: 10.1128/JB.00365-20. Print 2020 Nov 19.
Affiliations
- PMID: 32868406
- PMCID: PMC7685563
- DOI: 10.1128/JB.00365-20
Identification of Uncharacterized Components of Prokaryotic Immune Systems and Their Diverse Eukaryotic Reformulations
A Maxwell Burroughs et al. J Bacteriol. 2020.
Abstract
Nucleotide-activated effector deployment, prototyped by interferon-dependent immunity, is a common mechanistic theme shared by immune systems of several animals and prokaryotes. Prokaryotic versions include CRISPR-Cas with the CRISPR polymerase domain, their minimal variants, and systems with second messenger oligonucleotide or dinucleotide synthetase (SMODS). Cyclic or linear oligonucleotide signals in these systems help set a threshold for the activation of potentially deleterious downstream effectors in response to invader detection. We establish such a regulatory mechanism to be a more general principle of immune systems, which can also operate independently of such messengers. Using sensitive sequence analysis and comparative genomics, we identify 12 new prokaryotic immune systems, which we unify by this principle of threshold-dependent effector activation. These display regulatory mechanisms paralleling physiological signaling based on 3'-5' cyclic mononucleotides, NAD+-derived messengers, two- and one-component signaling that includes histidine kinase-based signaling, and proteolytic activation. Furthermore, these systems allowed the identification of multiple new sensory signal sensory components, such as a tetratricopeptide repeat (TPR) scaffold predicted to recognize NAD+-derived signals, unreported versions of the STING domain, prokaryotic YEATS domains, and a predicted nucleotide sensor related to receiver domains. We also identify previously unrecognized invader detection components and effector components, such as prokaryotic versions of the Wnt domain. Finally, we show that there have been multiple acquisitions of unidentified STING domains in eukaryotes, while the TPR scaffold was incorporated into the animal immunity/apoptosis signal-regulating kinase (ASK) signalosome.IMPORTANCE Both prokaryotic and eukaryotic immune systems face the dangers of premature activation of effectors and degradation of self-molecules in the absence of an invader. To mitigate this, they have evolved threshold-setting regulatory mechanisms for the triggering of effectors only upon the detection of a sufficiently strong invader signal. This work defines general templates for such regulation in effector-based immune systems. Using this, we identify several previously uncharacterized prokaryotic immune mechanisms that accomplish the regulation of downstream effector deployment by using nucleotide, NAD+-derived, two-component, and one-component signals paralleling physiological homeostasis. This study has also helped identify several previously unknown sensor and effector modules in these systems. Our findings also augment the growing evidence for the emergence of key animal immunity and chromatin regulatory components from prokaryotic progenitors.
Keywords: ASK signalosome; NAD+; SMODS; TPR-S; Wnts; YEATS; biological conflict systems; cyclic nucleotides; histidine kinase; sensor domain.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Figures
FIG 1
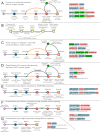
Discovery and generalized mechanisms of systems described in this study. (A) Generalized conceptual diagram of the interactions between core components of previously described nucleotide-activated effector conflict systems. Components are shown as nodes connected by arrows representing interactions. Orange lines are reserved for interactions mediated by second messenger diffusion. Dotted lines indicate predicted interactions. Examples of components are listed below or to the right of the nodes. Example gene neighborhoods are provided to the right of the diagram. Genes are depicted as boxed arrows, with the arrowhead pointing in the genome 3′ direction. Gene colors match the coloring of diagram components. GenBank accession numbers and organism names are provided as labels below each neighborhood. Enzymatic domains predicted to be catalytically inactive are marked by a red “X.” (B) Flowchart showing the process used to identify novel conflict systems. (C to G) Generalized conceptual diagrams depicting novel discoveries in nucleotide-activated effector conflict systems (C), systems dependent on NAD+-derived signals (D), systems dependent on caspase-catalyzed proteolysis (E), systems dependent on a core histidine kinase signaling module (F), and single-component sensing and effector activation systems (G). Flat arrowheads depict repressing interactions in panel E. Otherwise, the depictions are as described above for panel A.
FIG 2
Novel components and examples in nucleotide-activated effector conflict systems. (A to D) Representative conserved gene neighborhoods, as labeled. Gene neighborhood depictions are as described in the legend to Fig. 1A. Conserved components of a neighborhood with no cognate functional node in Fig. 1 are shown in gray. (E) Multiple-sequence alignment of the STING domain. Sequences are labeled to the left by organism abbreviation (see the supplemental material) and GenBank accession number. The secondary structure is shown on the top line, and the consensus abbreviation/coloring on the bottom line are as follows: s, small/green; u, tiny/green; l, aliphatic/yellow; h, hydrophobic/yellow; a, aromatic/yellow; p, polar/blue; b, big/gray. Nucleotide-binding residues are shaded in black and colored in white, marked by an asterisk at the top of the alignment. A caret marks the position of importance in eukaryotic STING but of limited conservation in prokaryotes. (F) Structural rendering of the STING dimer. Conserved residues are rendered in ball-and-stick form and shown relative to the cyclic dinucleotide ligand. (G) Conserved domain architectures for the STING sensor fused to diverse effector domains. Domains in architectures are depicted as distinct shapes, with the organism name and GenBank accession number provided. (H and I) Conserved gene neighborhoods as labeled and described in the legend to Fig. 1A.
FIG 3
Identification and characterization of the TPR-S domain and its NAD+-derived nucleotide-activated effector conflict systems. (A) Multiple-sequence alignment of the TPR-S domain, as described in the legend to Fig. 2E. Conserved residues with a predicted role in substrate recognition are denoted with asterisks above the alignment and shaded in black. α-Helical hairpin TPR repeats are labeled above the alignment. (B and C) Structural rendering of the TPR-S domain, in side view (B) and top view (C). Individual helices are color-coordinated with the secondary structure in panel A. (D) Surface rendering of the TPR-S domain, in top view as shown in panel C. Surfaces of absolutely conserved tryptophan and asparagine residues are shown in orange and green, respectively. Other conserved polar and aromatic residues are shown in red and yellow, respectively. (E to P) Representative conserved gene neighborhoods and domain architectures of NAD+-derived nucleotide-activated conflict systems, as labeled. Depictions are as described in the legends to Fig. 1A and Fig. 2G. C. violaceum, Chromobacterium violaceum; L. maritimus, Lutibacter maritimus; C. bacterium, “Candidatus Cloacimonetes bacterium”; C. limnaeum, Chlorobaculum limnaeum.
FIG 4
(A to G) Representative conserved gene neighborhoods and domain architectures of CATRA (A)- and histidine kinase (B and C)-mediated and pYEATS-centered (D to G) effector conflict systems. Depictions are as described in the legends to Fig. 1A and Fig. 2G. (H) Multiple-sequence alignment of the pYEATS domain, as described in the legend to Fig. 2E. Conserved residues contributing to the “aromatic cage” are shaded in black. (I) Structural rendering of the eukaryotic YEATS domain bound to crotonylated lysine. Conserved residues shared with the pYEATS domain are rendered as space filled and labeled. (J to M) Representative conserved gene neighborhoods for new components of prokaryotic counterinvader systems, as described in the legend to Fig. 1A. (N and O) Representative domain architectures for eukaryotic STING (N) and TPR-S (O) domain acquisitions, as described in the legend to Fig. 2G. C. H. archaeon, “Candidatus Heimdallarchaeota archaeon.”
FIG 5
Generalized conceptual diagrams comparing nucleotide-activated effector conflict and physiological signaling systems. Diagrams are as described in the legend to Fig. 1. Example domains and functional categories for both classical nucleotide and NAD+-derived second messenger systems are provided. Domains shared across the two classes are shown in red.
Comment in
- The Linguistics of Bacterial Conflict Systems Reveal Ancient Origins of Eukaryotic Innate Immunity.
Kibby EM, Whiteley AT. Kibby EM, et al. J Bacteriol. 2020 Nov 19;202(24):e00507-20. doi: 10.1128/JB.00507-20. Print 2020 Nov 19. J Bacteriol. 2020. PMID: 32958633 Free PMC article.
Similar articles
- Bacterial death and TRADD-N domains help define novel apoptosis and immunity mechanisms shared by prokaryotes and metazoans.
Kaur G, Iyer LM, Burroughs AM, Aravind L. Kaur G, et al. Elife. 2021 Jun 1;10:e70394. doi: 10.7554/eLife.70394. Elife. 2021. PMID: 34061031 Free PMC article. - Comparative genomic analyses reveal a vast, novel network of nucleotide-centric systems in biological conflicts, immunity and signaling.
Burroughs AM, Zhang D, Schäffer DE, Iyer LM, Aravind L. Burroughs AM, et al. Nucleic Acids Res. 2015 Dec 15;43(22):10633-54. doi: 10.1093/nar/gkv1267. Epub 2015 Nov 20. Nucleic Acids Res. 2015. PMID: 26590262 Free PMC article. - Polymorphic toxin systems: Comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics.
Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. Zhang D, et al. Biol Direct. 2012 Jun 25;7:18. doi: 10.1186/1745-6150-7-18. Biol Direct. 2012. PMID: 22731697 Free PMC article. - Diversity, evolution, and therapeutic applications of small RNAs in prokaryotic and eukaryotic immune systems.
Cooper EL, Overstreet N. Cooper EL, et al. Phys Life Rev. 2014 Mar;11(1):113-34. doi: 10.1016/j.plrev.2013.11.002. Epub 2013 Nov 8. Phys Life Rev. 2014. PMID: 24286719 Review. - Versatile modes of cellular regulation via cyclic dinucleotides.
Krasteva PV, Sondermann H. Krasteva PV, et al. Nat Chem Biol. 2017 Mar 22;13(4):350-359. doi: 10.1038/nchembio.2337. Nat Chem Biol. 2017. PMID: 28328921 Free PMC article. Review.
Cited by
- Conservation and similarity of bacterial and eukaryotic innate immunity.
Ledvina HE, Whiteley AT. Ledvina HE, et al. Nat Rev Microbiol. 2024 Jul;22(7):420-434. doi: 10.1038/s41579-024-01017-1. Epub 2024 Feb 28. Nat Rev Microbiol. 2024. PMID: 38418927 Free PMC article. Review. - Jumbo Phages: A Comparative Genomic Overview of Core Functions and Adaptions for Biological Conflicts.
M Iyer L, Anantharaman V, Krishnan A, Burroughs AM, Aravind L. M Iyer L, et al. Viruses. 2021 Jan 5;13(1):63. doi: 10.3390/v13010063. Viruses. 2021. PMID: 33466489 Free PMC article. - Apprehending the NAD+-ADPr-Dependent Systems in the Virus World.
Iyer LM, Burroughs AM, Anantharaman V, Aravind L. Iyer LM, et al. Viruses. 2022 Sep 7;14(9):1977. doi: 10.3390/v14091977. Viruses. 2022. PMID: 36146784 Free PMC article. - Reappraisal of the DNA phosphorothioate modification machinery: uncovering neglected functional modalities and identification of new counter-invader defense systems.
Rakesh S, Aravind L, Krishnan A. Rakesh S, et al. Nucleic Acids Res. 2024 Feb 9;52(3):1005-1026. doi: 10.1093/nar/gkad1213. Nucleic Acids Res. 2024. PMID: 38163645 Free PMC article. - The Linguistics of Bacterial Conflict Systems Reveal Ancient Origins of Eukaryotic Innate Immunity.
Kibby EM, Whiteley AT. Kibby EM, et al. J Bacteriol. 2020 Nov 19;202(24):e00507-20. doi: 10.1128/JB.00507-20. Print 2020 Nov 19. J Bacteriol. 2020. PMID: 32958633 Free PMC article.
References
- Smith JM, Price GR. 1973. The logic of animal conflict. Nature 246:15–18. doi:10.1038/246015a0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials