COVID-19 is, in the end, an endothelial disease - PubMed (original) (raw)
Review
COVID-19 is, in the end, an endothelial disease
Peter Libby et al. Eur Heart J. 2020.
Abstract
The vascular endothelium provides the crucial interface between the blood compartment and tissues, and displays a series of remarkable properties that normally maintain homeostasis. This tightly regulated palette of functions includes control of haemostasis, fibrinolysis, vasomotion, inflammation, oxidative stress, vascular permeability, and structure. While these functions participate in the moment-to-moment regulation of the circulation and coordinate many host defence mechanisms, they can also contribute to disease when their usually homeostatic and defensive functions over-reach and turn against the host. SARS-CoV-2, the aetiological agent of COVID-19, causes the current pandemic. It produces protean manifestations ranging from head to toe, wreaking seemingly indiscriminate havoc on multiple organ systems including the lungs, heart, brain, kidney, and vasculature. This essay explores the hypothesis that COVID-19, particularly in the later complicated stages, represents an endothelial disease. Cytokines, protein pro-inflammatory mediators, serve as key danger signals that shift endothelial functions from the homeostatic into the defensive mode. The endgame of COVID-19 usually involves a cytokine storm, a phlogistic phenomenon fed by well-understood positive feedback loops that govern cytokine production and overwhelm counter-regulatory mechanisms. The concept of COVID-19 as an endothelial disease provides a unifying pathophysiological picture of this raging infection, and also provides a framework for a rational treatment strategy at a time when we possess an indeed modest evidence base to guide our therapeutic attempts to confront this novel pandemic.
Keywords: Inflammation; Microvasculature; Thrombosis; Cytokine; Endothelium.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2020. For permissions, please email: journals.permissions@oup.com.
Figures
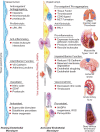
Figure 1
The left side of the diagram depicts a resting endothelial monolayer with the endothelial cells of squamous morphology resting on an intact basement membrane. The homeostatic mechanisms displayed by the resting endothelium include the listed properties as detailed in the text. When the endothelial cells undergo the cytopathic effect of a viral infection such as SARS-CoV-2, or encounter pathogen-associated molecular patterns (PAMPs) derived from viruses or bacteria such as lipopolysaccharide, proinflammatory cytokines such as IL-1 or TNF, or damage-associated molecular patterns (DAMPs) derived from dead or dying cells, the endothelial cells become activated. The endothelial cells display more columnar morphology. They can express adhesion molecules that attract leucocytes and chemokines that direct their migration into the subendothelial space. Sloughing of endothelial cells uncovers the thrombogenic basement membrane. Adherent neutrophils can undergo formation of neutrophil extracellular traps that provide an amplifier for endothelial damage mediated in part by IL-1α. Inflammatory activation of endothelial cells can disrupt VE-cadherin largely responsible for the integrity of the endothelial barrier function. Activated endothelial cells can also express matrix metalloproteinases that can degrade the basement membrane and further interrupt endothelial barrier function. In small vessels, such as those that embrace alveoli in the lung, this impaired barrier function can lead to capillary leak. These various disturbances in endothelial function, depicted in the middle part of the diagram, lead to end organ damage including adult respiratory distress syndrome and thrombosis in the lungs, predispose to plaque rupture and thrombosis in coronary arteries, and affect the microvasculature leading to myocardial ischaemia and damage. The thrombotic diathesis provoked by endothelial dysfunction can also predispose towards strokes. Microvascular as well macrovascular injury can potentiate acute renal failure. Hepatic dysfunction can also result from microvascular thrombosis among other mechanisms. Deep venous thrombosis can occur as endothelial disfunction represents an important part of Virchow’s triad, and sets the stage for pulmonary embolism. Thus, loss of the endothelial protective and unleashing of the mechanisms depicted can lead to multiorgan system failure that characterizes the advanced stages of COVID-19.
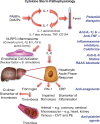
Figure 2
Cytokine storm. Proinflammatory cytokines such as IL-1 and TNF-α induce each other’s gene expression, unleashing an amplification loop that sustains the cytokine storm. The endothelial cell is a key target of cytokines, as they induce action of a central proinflammatory transcriptional hub, nuclear factor-κB. IL-1 also causes substantial increases in production by endothelial and other cells of IL-6, the instigator of the hepatocyte acute phase response. The acute phase reactants include fibrinogen, the precursor of clot, and PAI-1, the major inhibitor of our endogenous fibrinolytic system. C-reactive protein, commonly elevated in COVID-19, provides a readily measured biomarker of inflammatory status. The alterations in the thrombotic/fibrinolytic balance due to the acute phase response promotes thrombosis in arteries, in the microvasculature including that of organs such as the myocardium and kidney, and in veins, causing deep vein thrombosis and predisposing towards pulmonary embolism. Thus, the very same cytokines that elicit abnormal endothelial functions can unleash the acute phase response which together with local endothelial disfunction can conspire to cause the clinical complications of COVID-19. The right side of this diagram aligns therapeutic agents that attack these mechanisms of the cytokine storm and may thus limit its devastating consequences.
Comment in
- Understanding COVID-19: in the end it is the endothelium-what else?
Lüscher TF. Lüscher TF. Eur Heart J. 2020 Aug 21;41(32):3023-3027. doi: 10.1093/eurheartj/ehaa706. Eur Heart J. 2020. PMID: 33216863 Free PMC article. No abstract available.
Similar articles
- Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy.
Zhang J, Tecson KM, McCullough PA. Zhang J, et al. Rev Cardiovasc Med. 2020 Sep 30;21(3):315-319. doi: 10.31083/j.rcm.2020.03.126. Rev Cardiovasc Med. 2020. PMID: 33070537 Review. - COVID-19 May Predispose to Thrombosis by Affecting Both Vascular Endothelium and Platelets.
Cure E, Cure MC. Cure E, et al. Clin Appl Thromb Hemost. 2020 Jan-Dec;26:1076029620933945. doi: 10.1177/1076029620933945. Clin Appl Thromb Hemost. 2020. PMID: 32619104 Free PMC article. No abstract available. - The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection.
Pons S, Fodil S, Azoulay E, Zafrani L. Pons S, et al. Crit Care. 2020 Jun 16;24(1):353. doi: 10.1186/s13054-020-03062-7. Crit Care. 2020. PMID: 32546188 Free PMC article. Review. - Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19?
Panigrahy D, Gilligan MM, Huang S, Gartung A, Cortés-Puch I, Sime PJ, Phipps RP, Serhan CN, Hammock BD. Panigrahy D, et al. Cancer Metastasis Rev. 2020 Jun;39(2):337-340. doi: 10.1007/s10555-020-09889-4. Cancer Metastasis Rev. 2020. PMID: 32385712 Free PMC article. - Productivity of Young Experts in Hemostasis and Thrombosis before and during the SARS-CoV-2 Pandemic-A Special Issue.
Scharf RE. Scharf RE. Hamostaseologie. 2020 Aug;40(3):255-256. doi: 10.1055/a-1081-6973. Epub 2020 Jul 29. Hamostaseologie. 2020. PMID: 32726820 No abstract available.
Cited by
- Infection and pulmonary vascular diseases consortium: United against a global health challenge.
Oliveira SD, Almodóvar S, Butrous G, De Jesus Perez V, Fabro A, Graham BB, Mocumbi A, Nyasulu PS, Tura-Ceide O, Oliveira RKF, Dhillon NK; Infection and Pulmonary Vascular Diseases Consortium. Oliveira SD, et al. Pulm Circ. 2024 Nov 12;14(4):e70003. doi: 10.1002/pul2.70003. eCollection 2024 Oct. Pulm Circ. 2024. PMID: 39534510 Free PMC article. - Exploring the Therapeutic Potential of Vitamin D in Kawasaki Disease and Its Interplay with the COVID-19.
Visuddho V, Welliam Y, Maitri Aldian F, Tri Arif Sampurna M, Irzaldy A. Visuddho V, et al. Turk Arch Pediatr. 2024 Sep 2;59(5):432-439. doi: 10.5152/TurkArchPediatr.2024.24141. Turk Arch Pediatr. 2024. PMID: 39439407 Free PMC article. - Central artery pulse pressure, not central arterial stiffness impact on all-cause mortality in patients with viral pneumonia infection.
Jin L, Chen J, Wu L, Zhang M, Tang X, Shen C, Sun J, Du L, Wang X, Li Z. Jin L, et al. BMC Infect Dis. 2024 Oct 21;24(1):1183. doi: 10.1186/s12879-024-10091-y. BMC Infect Dis. 2024. PMID: 39434023 Free PMC article. - The home-based breathing and chest mobility exercise improves cardiorespiratory functional capacity in long COVID with cardiovascular comorbidities: a randomized study.
Dwiputra B, Ambari AM, Triangto K, Supriami K, Kesuma TW, Zuhdi N, Phowira J, Radi B. Dwiputra B, et al. BMC Cardiovasc Disord. 2024 Oct 18;24(1):574. doi: 10.1186/s12872-024-04196-0. BMC Cardiovasc Disord. 2024. PMID: 39425012 Free PMC article. Clinical Trial. - Arterial Thrombosis in Acute Respiratory Infections: An Underestimated but Clinically Relevant Problem.
Babkina AS, Pisarev MV, Grechko AV, Golubev AM. Babkina AS, et al. J Clin Med. 2024 Oct 9;13(19):6007. doi: 10.3390/jcm13196007. J Clin Med. 2024. PMID: 39408067 Free PMC article. Review.
References
- Aird WC. Endothelium. In: Kitchens CS, Kessler CM, Konkle BA, eds. Consultative Hemostasis and Thrombosis, 3rd ed. Philadelphia. PA: W.B. Saunders; 2013. p33–41.
- Libby P. The vascular biology of atherosclerosis. In: Zipes DP, Libby P, Bonow RO, Mann DL, Tomaselli GF, eds. Braunwald’s Heart Disease, 11th ed. Philadelphia, PA: Elsevier; 2018. p859–875.
- Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol 2007;7:803–815. - PubMed
- Libby P. The active roles of cells of the blood vessel wall in health and disease. Mol Aspects Med 1987;9:499–567. - PubMed
- Libby P, d Birinyi LK. The dynamic nature of vascular endothelial functions. In: Zilla P, Fasol R, Deutsch M, eds. Endothelialization of Vascular Grafts. Basel: Karger; 1987. p80–99.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous