Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets - PubMed (original) (raw)
doi: 10.1186/s40104-020-00496-5. eCollection 2020.
Yanyan Song # 1 2, Bing Yu 1 2, Jun He 1 2, Ping Zheng 1 2, Xiangbing Mao 1 2, Zhiqing Huang 1 2, Yuheng Luo 1 2, Junqiu Luo 1 2, Hui Yan 1 2, Quyuan Wang 1 2, Huifen Wang 1 2, Daiwen Chen 1 2
Affiliations
- PMID: 32884745
- PMCID: PMC7460753
- DOI: 10.1186/s40104-020-00496-5
Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets
Jie Yu et al. J Anim Sci Biotechnol. 2020.
Abstract
Background: Tannic acid (TA) is potential to reduce diarrhea in weaning pigs, but knowledge about the influence of TA on intestinal barrier integrity and function is still scarce. This experiment was conducted to investigate the effects of dietary TA supplementation on growth performance, diarrhea rate, intestinal barrier integrity and function of weaned pigs.
Methods: A total of 108 crossbred (Duroc × Landrace × Yorkshire) piglets, with an initial average body weight of 6.60 ± 0.27 kg, were allotted to 3 groups (6 pigs/pen and 6 replicates/group) in a randomized complete block design according to their gender and body weight. Piglets were fed the basal diet with 0 (control, CON), 0.2% and 1.0% TA, respectively. The trial lasted for 28 d.
Results: Compared with the CON group, dietary 0.2% and 1.0% TA supplementation didn't affect ADFI, ADG and F:G (P > 0.05), but reduced diarrhea rate, diarrhea index and diarrhea score of piglets (P < 0.05), reduced diamine oxidase (DAO) activity and D-lactic acid concentration in serum (P < 0.01). The higher occludin expression and localization were observed in the duodenum, jejunum and ileum after supplementation with 0.2% or 1.0% TA (P < 0.05). Adding 0.2% TA to diet significantly decreased crypt depth, increased villus height/crypt depth ratio in the duodenum (P < 0.05), and dietary 1.0% TA tended to decrease crypt depth (P < 0.10) and significantly decreased villus height (P < 0.05) of the ileum. Moreover, lower malondialdehyde content in the ileum was detected in the pigs fed 1.0% TA (P < 0.05). In the duodenum, both 0.2% and 1.0% TA groups had higher occludin (OCLN) mRNA and 0.2% TA group had higher zonula occludens-2 (ZO-2) level (P < 0.05). Meanwhile, dietary 1.0% TA supplementation tended to up-regulate OCLN mRNA levels in the jejunum (P < 0.10) and 0.2% TA supplementation tended to up-regulate zonula occludens-1 (ZO-1) mRNA levels in the ileum (P < 0.10).
Conclusion: In conclusion, dietary supplementation of 0.2% or 1.0% TA could effectively alleviate post-weaning diarrhea without altering growth performance in weaned piglets, which might be achieved by improving intestinal barrier integrity and function.
Keywords: Intestine barrier; Post-weaning diarrhea; Tannic acid; Weaned piglets.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsAll authors declare that they have no competing interests.
Figures
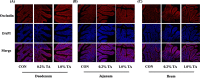
Fig. 1
Effect of tannic acid (TA) on expression and localization of occludin protein in small intestine of weaned piglets (scale bar: 100 μm). The localization of tight junction protein occludin in duodenum (a), jejunum (b) and ileum (c) of weaned piglets was visualized using immunofluorescence technique. The localization of occludin (red), DAPI (blue), as well as merged occludin and DAPI are shown. DAPI stain indicates live cells. CON, piglets receiving a basal diet; CON + 0.2% TA, piglets receiving a basal diet supplemented with 0.2% TA; CON + 1.0% TA, piglets receiving a basal diet supplemented with 1.0% TA
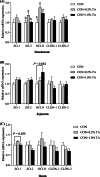
Fig. 2
Effect of tannic acid (TA) on mRNA levels of tight junction protein-related genes in small intestine of weaned piglets. The mRNA expressions of tight junction protein-related genes in duodenum (a), jejunum (b), and ileum (c) of weaned piglets. CON, piglets receiving a basal diet; CON + 0.2% TA, piglets receiving a basal diet supplemented with 0.2% TA; CON + 1.0% TA, piglets receiving a basal diet supplemented with 1.0% TA; ZO-1, zonula occludens 1; ZO-2, zonula occludens 2; OCLN, occludin; CLDN-1, claudin 1; CLDN-2, claudin 2. The values shown represent the means ± SEM, n = 6; a,bMean values with unlike superscript letters were significantly different (P < 0.05)
Similar articles
- Tannic acid extracted from gallnut prevents post-weaning diarrhea and improves intestinal health of weaned piglets.
Song Y, Luo Y, Yu B, He J, Zheng P, Mao X, Huang Z, Luo J, Luo Y, Yan H, Wang Q, Wang H, Chen D, Yu J. Song Y, et al. Anim Nutr. 2021 Dec;7(4):1078-1086. doi: 10.1016/j.aninu.2021.04.005. Epub 2021 Sep 14. Anim Nutr. 2021. PMID: 34738038 Free PMC article. - Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets.
Xiong X, Yang HS, Wang XC, Hu Q, Liu CX, Wu X, Deng D, Hou YQ, Nyachoti CM, Xiao DF, Yin YL. Xiong X, et al. J Anim Sci. 2015 Mar;93(3):1089-97. doi: 10.2527/jas.2014-7851. J Anim Sci. 2015. PMID: 26020885 - Dietary Lactobacillus rhamnosus GG Supplementation Improves the Mucosal Barrier Function in the Intestine of Weaned Piglets Challenged by Porcine Rotavirus.
Mao X, Gu C, Hu H, Tang J, Chen D, Yu B, He J, Yu J, Luo J, Tian G. Mao X, et al. PLoS One. 2016 Jan 4;11(1):e0146312. doi: 10.1371/journal.pone.0146312. eCollection 2016. PLoS One. 2016. PMID: 26727003 Free PMC article. - Dietary nutrition, intestinal microbiota dysbiosis and post-weaning diarrhea in piglets.
Han X, Hu X, Jin W, Liu G. Han X, et al. Anim Nutr. 2024 Feb 28;17:188-207. doi: 10.1016/j.aninu.2023.12.010. eCollection 2024 Jun. Anim Nutr. 2024. PMID: 38800735 Free PMC article. Review. - Diarrhea induced by insufficient fat absorption in weaned piglets: Causes and nutrition regulation.
Li Y, Shi P, Yao K, Lin Q, Wang M, Hou Z, Tang W, Diao H. Li Y, et al. Anim Nutr. 2023 Dec 27;16:299-305. doi: 10.1016/j.aninu.2023.12.004. eCollection 2024 Mar. Anim Nutr. 2023. PMID: 38371473 Free PMC article. Review.
Cited by
- Supplementation of curcumin promotes the intestinal structure, immune barrier function and cecal microbiota composition of laying hens in early laying period.
Xu Z, Zhu W, Xu D, Amevor FK, Wu Y, Ma D, Cao X, Wei S, Shu G, Zhao X. Xu Z, et al. Poult Sci. 2024 Sep 24;103(12):104355. doi: 10.1016/j.psj.2024.104355. Online ahead of print. Poult Sci. 2024. PMID: 39423789 Free PMC article. - Terminalia Chebula Extract Replacing Zinc Oxide Enhances Antioxidant and Anti-Inflammatory Capabilities, Improves Growth Performance, and Promotes Intestinal Health in Weaned Piglets.
Wang T, Li Y, Yin L, Chen J, Shi P, Wang F, Wu K, Yao K, Yin Y. Wang T, et al. Antioxidants (Basel). 2024 Sep 5;13(9):1087. doi: 10.3390/antiox13091087. Antioxidants (Basel). 2024. PMID: 39334746 Free PMC article. - Crude Blueberry Phenolic Extracts Improve Gut Barrier Integrity and Exert Anti-Inflammatory and Antimicrobial Activity in an In Vitro Weaning Stress Model.
Nathan VB, Eckrote S, Li S, Reddivari L. Nathan VB, et al. Antioxidants (Basel). 2024 Aug 28;13(9):1044. doi: 10.3390/antiox13091044. Antioxidants (Basel). 2024. PMID: 39334703 Free PMC article. - Research progress on cottonseed meal as a protein source in pig nutrition: An updated review.
Tao A, Wang J, Luo B, Liu B, Wang Z, Chen X, Zou T, Chen J, You J. Tao A, et al. Anim Nutr. 2024 May 25;18:220-233. doi: 10.1016/j.aninu.2024.03.020. eCollection 2024 Sep. Anim Nutr. 2024. PMID: 39281049 Free PMC article. Review. - Dietary xylo-oligosaccharides alleviates LPS-induced intestinal injury via endoplasmic reticulum-mitochondrial system pathway in piglets.
Liu G, Sun W, Zhang R, Shen F, Jia G, Zhao H, Chen X, Wang J. Liu G, et al. J Anim Sci. 2024 Jan 3;102:skae238. doi: 10.1093/jas/skae238. J Anim Sci. 2024. PMID: 39155504
References
- Ye M-H, Nan Y-L, Ding M-M, Hu J-B, Liu Q, Wei W-H, et al. Effects of dietary tannic acid on the growth, hepatic gene expression, and antioxidant enzyme activity in Brandt's voles (Microtus brandti). Comp Biochem Physiol B Biochem Mol Biol. 2016;196:19–26. - PubMed
- Mueller-Harvey I. Unravelling the conundrum of tannins in animal nutrition and health. J Sci Food Agric. 2006;86(13):2010–2037. doi: 10.1002/jsfa.2577. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous