Hierarchical Capillary Coating to Biofunctionlize Drug-Eluting Stent for Improving Endothelium Regeneration - PubMed (original) (raw)
Hierarchical Capillary Coating to Biofunctionlize Drug-Eluting Stent for Improving Endothelium Regeneration
Jing Wang et al. Research (Wash D C). 2020.
Abstract
The drug-eluting stent (DES) has become one of the most successful and important medical devices for coronary heart disease, but yet suffers from insufficient endothelial cell (EC) growth and intima repair, eventually leading to treatment failure. Although biomacromolecules such as vascular endothelial growth factor (VEGF) would be promising to promote the intima regeneration, combining hydrophilic and vulnerable biomacromolecules with hydrophobic drugs as well as preserving the bioactivity after harsh treatments pose a huge challenge. Here, we report on a design of hierarchical capillary coating, which composes a base solid region and a top microporous region for incorporating rapamycin and VEGF, respectively. The top spongy region can guarantee the efficient, safe, and controllable loading of VEGF up to 1 _μ_g/cm2 in 1 minute, providing a distinctive real-time loading capacity for saving the bioactivity. Based on this, we demonstrate that our rapamycin-VEGF hierarchical coating impressively promoted the competitive growth of endothelial cells over smooth muscle cells (ratio of EC/SMC~25) while relieving the adverse impact of rapamycin to ECs. We further conducted the real-time loading of VEGF on stents and demonstrate that the hierarchical combination of rapamycin and VEGF showed remarkable endothelium regeneration while maintaining a very low level of in-stent restenosis. This work paves an avenue for the combination of both hydrophobic and hydrophilic functional molecules, which should benefit the next generation of DES and may extend applications to diversified combination medical devices.
Copyright © 2020 Jing Wang et al.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Scheme 1
Schematic illustration of the hierarchical coating on the cardiovascular stent for regulating the intimal regeneration. The capillary-based wicking action could realize the distinctive real-time loading of VEGF during the surgery, which preserved the bioactivity of VEGF and impressively enhanced endothelium recovery while maintaining the inhibition of SMC proliferation after implantation.
Figure 1
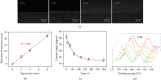
Preparation of the hierarchical coating. (a) SEM micrographs of the coating with different top layer spray time. (b) The thickness of the top layer as a function of spray time. Contact angle (c) and X-ray photoelectron spectrums (d) of the coating with different extent of heparinization based on UV irradiation.
Figure 2
The loading and release profile of the hierarchical coating. (a) Rapamycin release profile as a function of time. (b) Comparison of rapamycin release with and without spongy top layer, respectively. (c) The wicking action of rhodamine B. (d) Loading of VEGF as a function of VEGF solution concentration. (e) VEGF release profile as a function of time.
Figure 3
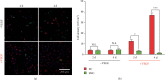
Coculture of ECs with SMCs. Confocal micrographs (a) and corresponding cell density (b) of ECs and SMCs on the hierarchical coatings (n = 5, ∗P < 0.05, ∗∗∗P < 0.001).
Figure 4
Relative expression of endothelial function related genes. Values normalized to control samples (n = 3, ∗P < 0.05).
Figure 5
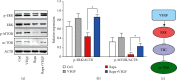
Regulation of the m-TOR signaling in ECs via the spatial combination of VEGF with rapamycin. (a) Western blot for ERK1/2 and its downstream effector m-TOR. (b) Quantification of Western blot bands. Values normalized to ACTB. (c) Potential signaling pathway related to the regulation of m-TOR by VEGF (n = 3, ∗P < 0.05).
Figure 6
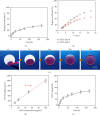
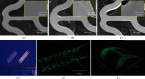
Construction of hierarchical coating onto the stents: SEM micrographs of BSs (a), RESs (b), and VRSs (c). (d) Digital photo of BSs (left) and VRSs (right). Fluorescence micrographs (e) and confocal scanning (f) of VRSs after loading of PLL-FITC.
Figure 7
Histological analysis results at 6 weeks. (a) The digital photos of the stent with a hierarchical coating (left) and the typical wicking process during the surgery (right). (b) The micrographs of the H&E-stained cross-section slices of the arteries with BSs, RESs, and VRSs, respectively. Histological analysis of neointimal thickness (c), and the percentage of neointimal stenosis (d) (n = 3, ∗P < 0.05).
Figure 8
Immunohistochemical analysis of the stented arterial segments for CD31 and Col-I, respectively.
Figure 9
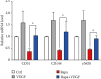
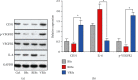
(a) Western blot analysis of arterial segments implanted with BSs, RESs, and VRSs, respectively. Ctrl represents the native arterial segments. (b) Quantification of Western blot bands. Values normalized to GAPDH and then normalized to Ctrl (n = 3, ∗P < 0.05).
Similar articles
- miR-22 eluting cardiovascular stent based on a self-healable spongy coating inhibits in-stent restenosis.
Wang J, Qian HL, Chen SY, Huang WP, Huang DN, Hao HY, Ren KF, Wang YB, Fu GS, Ji J. Wang J, et al. Bioact Mater. 2021 May 20;6(12):4686-4696. doi: 10.1016/j.bioactmat.2021.04.037. eCollection 2021 Dec. Bioact Mater. 2021. PMID: 34095625 Free PMC article. - Clinical Effectiveness and Cost Effectiveness of Intracoronary Brachytherapy and Drug Eluting Stents [Internet].
Mørland B, Kløw NE, Rotevatn S, Steigen T, Vatne K, Wisløff T, Kristiansen IS, Norderhaug I. Mørland B, et al. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2004. Report from Norwegian Knowledge Centre for the Health Services (NOKC) No. 08-2004. Oslo, Norway: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2004. Report from Norwegian Knowledge Centre for the Health Services (NOKC) No. 08-2004. PMID: 29320006 Free Books & Documents. Review. - REDV/Rapamycin-loaded polymer combinations as a coordinated strategy to enhance endothelial cells selectivity for a stent system.
Wei Y, Zhang JX, Ji Y, Ji J. Wei Y, et al. Colloids Surf B Biointerfaces. 2015 Dec 1;136:1166-73. doi: 10.1016/j.colsurfb.2015.11.012. Epub 2015 Nov 10. Colloids Surf B Biointerfaces. 2015. PMID: 26613858 - "Spongy skin" as a robust strategy to deliver 4-octyl itaconate for conducting dual-regulation against in-stent restenosis.
Qian HL, Chen SY, Jia F, Huang WP, Wang J, Ren KF, Fu GS, Ji J. Qian HL, et al. Biomaterials. 2023 May;296:122069. doi: 10.1016/j.biomaterials.2023.122069. Epub 2023 Feb 27. Biomaterials. 2023. PMID: 36893653 - Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting: approach to therapy.
Curcio A, Torella D, Indolfi C. Curcio A, et al. Circ J. 2011;75(6):1287-96. doi: 10.1253/circj.cj-11-0366. Epub 2011 Apr 29. Circ J. 2011. PMID: 21532177 Review.
Cited by
- Quenching the Macroporous Collapse of Polyelectrolyte Multilayer Films for Repeated Drug Loading.
Liang ZX, Li QS, Zhao ZK, Zhang D, Chen XC. Liang ZX, et al. ACS Omega. 2022 Apr 12;7(16):13853-13860. doi: 10.1021/acsomega.2c00204. eCollection 2022 Apr 26. ACS Omega. 2022. PMID: 35559176 Free PMC article. - Biomedical polymers: synthesis, properties, and applications.
Chen WH, Chen QW, Chen Q, Cui C, Duan S, Kang Y, Liu Y, Liu Y, Muhammad W, Shao S, Tang C, Wang J, Wang L, Xiong MH, Yin L, Zhang K, Zhang Z, Zhen X, Feng J, Gao C, Gu Z, He C, Ji J, Jiang X, Liu W, Liu Z, Peng H, Shen Y, Shi L, Sun X, Wang H, Wang J, Xiao H, Xu FJ, Zhong Z, Zhang XZ, Chen X. Chen WH, et al. Sci China Chem. 2022;65(6):1010-1075. doi: 10.1007/s11426-022-1243-5. Epub 2022 Apr 24. Sci China Chem. 2022. PMID: 35505924 Free PMC article. Review. - Immunomodulatory hybrid micro-nanofiber scaffolds enhance vascular regeneration.
Liu S, Yao L, Wang Y, Li Y, Jia Y, Yang Y, Li N, Hu Y, Kong D, Dong X, Wang K, Zhu M. Liu S, et al. Bioact Mater. 2022 Sep 18;21:464-482. doi: 10.1016/j.bioactmat.2022.08.018. eCollection 2023 Mar. Bioact Mater. 2022. PMID: 36185748 Free PMC article. - A "built-up" composite film with synergistic functionalities on Mg-2Zn-1Mn bioresorbable stents improves corrosion control effects and biocompatibility.
Dou Z, Chen S, Wang J, Xia L, Maitz MF, Tu Q, Zhang W, Yang Z, Huang N. Dou Z, et al. Bioact Mater. 2023 Feb 8;25:223-238. doi: 10.1016/j.bioactmat.2023.02.004. eCollection 2023 Jul. Bioact Mater. 2023. PMID: 36817823 Free PMC article. - Applying Principles of Regenerative Medicine to Vascular Stent Development.
Selvakumar PP, Rafuse MS, Johnson R, Tan W. Selvakumar PP, et al. Front Bioeng Biotechnol. 2022 Mar 7;10:826807. doi: 10.3389/fbioe.2022.826807. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35321023 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources