Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation - PubMed (original) (raw)
Review
Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation
Jing-Ru Liu et al. Cell Mol Life Sci. 2021 Feb.
Abstract
The gut microbiota has a crucial effect on regulating the intestinal mucosal immunity and maintaining intestinal homeostasis both in health and in disease state. Many effects are mediated by gut microbiota-derived metabolites and tryptophan, an essential aromatic amino acid, is considered important among many metabolites in the crosstalk between gut microbiota and the host. Kynurenine, serotonin, and indole derivatives are derived from the three major tryptophan metabolism pathways modulated by gut microbiota directly or indirectly. Aryl hydrocarbon receptor (AHR) is a cytoplasmic ligand-activated transcription factor involved in multiple cellular processes. Tryptophan metabolites as ligands can activate AHR signaling in various diseases such as inflammation, oxidative stress injury, cancer, aging-related diseases, cardiovascular diseases (CVD), and chronic kidney diseases (CKD). Accumulated uremic toxins in the body fluids of CKD patients activate AHR and affect disease progression. In this review, we will elucidate the relationship between gut microbiota-derived uremic toxins by tryptophan metabolism and AHR activation in CKD and its complications. This review will provide therapeutic avenues for targeting CKD and concurrently present challenges and opportunities for designing new therapeutic strategies against renal fibrosis.
Keywords: Chronic kidney disease; Intestinal flora; Natural products; Tryptophan metabolites.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
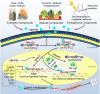
Fig. 1
The AHR signaling pathway. Inactive AHR is complexed with HSP90, XAP2, and p23 to maintain stability and keep it in a high-affinity state for its ligands. When the ligands bind to AHR, the complex translocates to the nucleus where it combines ARNT through HSP90 displacement. The AHR-ARNT heterodimer binds to XREs to induce the transcription of downstream target genes, such as CYP1A1, CYP1B1, AHRR, etc. AhRR is also a negative regulator of AHR that can bind to ARNT but does not induce transcription. Besides, the ligands–AHR complex also interacts with other regulator proteins, such as NF-κB, etc
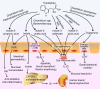
Fig. 2
Mechanisms of action of microbial tryptophan catabolites on host physiology and disease. Tryptophan in the colonic lumen is converted into various catabolites by the gut microbiota. Indole and indole-3-propionate (IPA) may mediate the pregnane X receptor (PXR) to decrease intestinal permeability and affect mucosal homeostasis. Indole is metabolized to indoxyl sulfate by CYP2E1 and sulfotransferases in the liver leading to the accumulation of IS, which is toxic and associated with renal dysfunction. Indole also induces the release of glucagon-like peptide 1 (GLP-1) in enteroendocrine L-cells, suppressing appetite and insulin secretion and slowing gastric emptying. Several tryptophan catabolites activate AHR in intestinal immune cells to alter innate and adaptive immune responses maintaining mucosal reactivity. In enterochromaffin cells, tryptamine induces the release of 5-HT stimulating gastrointestinal motility by acting on enteric nervous system neurons
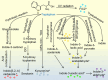
Fig. 3
AHR ligands from tryptophan metabolism. Tryptophan in cruciferous vegetables produce glucosinolate via hydrolysis reaction, yielding indolo[3,2,-b]carbazole. 95% tryptophan can be metabolized to kynurenine, which is mediated by IDO and TDO. In the gastrointestinal tract, metabolites such as tryptamine, indole-3-acetaldehyde, indole-3-acetic acid, and indole-3-aldehyde are from the microbial metabolic process. Besides, tryptophan can be metabolized to 6-formylindolo-(3,2-b)-carbazole from ultraviolet radiation. Asterisks indicate metabolites with AHR agonistic activity
Similar articles
- Activation of aryl hydrocarbon receptor (AhR) in Alzheimer's disease: role of tryptophan metabolites generated by gut host-microbiota.
Salminen A. Salminen A. J Mol Med (Berl). 2023 Mar;101(3):201-222. doi: 10.1007/s00109-023-02289-5. Epub 2023 Feb 9. J Mol Med (Berl). 2023. PMID: 36757399 Free PMC article. Review. - Aryl Hydrocarbon Receptor Activation in Chronic Kidney Disease: Role of Uremic Toxins.
Brito JS, Borges NA, Esgalhado M, Magliano DC, Soulage CO, Mafra D. Brito JS, et al. Nephron. 2017;137(1):1-7. doi: 10.1159/000476074. Epub 2017 May 11. Nephron. 2017. PMID: 28490014 Review. - The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease.
Sallée M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. Sallée M, et al. Toxins (Basel). 2014 Mar 4;6(3):934-49. doi: 10.3390/toxins6030934. Toxins (Basel). 2014. PMID: 24599232 Free PMC article. Review. - Host/microbiota interactions-derived tryptophan metabolites modulate oxidative stress and inflammation via aryl hydrocarbon receptor signaling.
Tan YQ, Wang YN, Feng HY, Guo ZY, Li X, Nie XL, Zhao YY. Tan YQ, et al. Free Radic Biol Med. 2022 May 1;184:30-41. doi: 10.1016/j.freeradbiomed.2022.03.025. Epub 2022 Mar 31. Free Radic Biol Med. 2022. PMID: 35367341 Review. - Aryl Hydrocarbon Receptor and Uremic Toxins from the Gut Microbiota in Chronic Kidney Disease Patients: Is There a Relationship between Them?
Brito JS, Borges NA, Anjos JSD, Nakao LS, Stockler-Pinto MB, Paiva BR, Cardoso-Weide LC, Cardozo LFMF, Mafra D. Brito JS, et al. Biochemistry. 2019 Apr 16;58(15):2054-2060. doi: 10.1021/acs.biochem.8b01305. Epub 2019 Apr 3. Biochemistry. 2019. PMID: 30912928
Cited by
- Coexposure to microplastic and Bisphenol A exhacerbates damage to human kidney proximal tubular cells.
Verzola D, Rumeo N, Alberti S, Loiacono F, La Maestra S, Passalacqua M, Artini C, Russo E, Verrina E, Angeletti A, Matarese S, Mancianti N, Cravedi P, Gentile M, Viazzi F, Esposito P, La Porta E. Verzola D, et al. Heliyon. 2024 Oct 15;10(20):e39426. doi: 10.1016/j.heliyon.2024.e39426. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39498083 Free PMC article. - _Tn_P and AHR-CYP1A1 Signaling Crosstalk in an Injury-Induced Zebrafish Inflammation Model.
Disner GR, Fernandes TAM, Nishiyama-Jr MY, Lima C, Wincent E, Lopes-Ferreira M. Disner GR, et al. Pharmaceuticals (Basel). 2024 Aug 31;17(9):1155. doi: 10.3390/ph17091155. Pharmaceuticals (Basel). 2024. PMID: 39338318 Free PMC article. - Hepatoprotective effects of magnolol in fatty liver hemorrhagic syndrome hens through shaping gut microbiota and tryptophan metabolic profile.
Lv Y, Ge C, Wu L, Hu Z, Luo X, Huang W, Zhan S, Shen X, Yu D, Liu B. Lv Y, et al. J Anim Sci Biotechnol. 2024 Sep 6;15(1):120. doi: 10.1186/s40104-024-01074-9. J Anim Sci Biotechnol. 2024. PMID: 39238062 Free PMC article. - Aryl Hydrocarbon Receptor Pathway Augments Peritoneal Fibrosis in a Murine CKD Model Exposed to Peritoneal Dialysate.
Lotfollahzadeh S, Vazirani A, Sellinger IE, Clovie J, Hoekstra I, Patel A, Malloum AB, Yin W, Paul H, Yadati P, Siracus J, Malikova M, Pernar LI, Francis J, Stern L, Chitalia VC. Lotfollahzadeh S, et al. Kidney360. 2024 Sep 1;5(9):1238-1250. doi: 10.34067/KID.0000000000000516. Epub 2024 Sep 5. Kidney360. 2024. PMID: 39235862 Free PMC article. - Interplay between gut microbiota and tryptophan metabolism in type 2 diabetic mice treated with metformin.
Xie Y, Li X, Meng Q, Li J, Wang X, Zhu L, Wang W, Li X. Xie Y, et al. Microbiol Spectr. 2024 Oct 3;12(10):e0029124. doi: 10.1128/spectrum.00291-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162538 Free PMC article.
References
- Corre S, Tardif N, Mouchet N, Leclair HM, Boussemart L, Gautron A, Bachelot L, Perrot A, Soshilov A, Rogiers A, Rambow F, Dumontet E, Tarte K, Bessede A, Guillemin GJ, Marine JC, Denison MS, Gilot D, Galibert MD. Sustained activation of the aryl hydrocarbon receptor transcription factor promotes resistance to BRAF-inhibitors in melanoma. Nat Commun. 2018;9(1):4775. doi: 10.1038/s41467-018-06951-2. - DOI - PMC - PubMed
- Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Rannug A, Törnqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002;110(Suppl 3):451–488. doi: 10.1289/ehp.110-1241197. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2019YFC1709405/National Key Research and Development Project of China
- 81872985/National Natural Science Foundation of China
- 81673578/National Natural Science Foundation of China
- 81603271/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical