Case 30-2020: A 54-Year-Old Man with Sudden Cardiac Arrest - PubMed (original) (raw)
Case Reports
Case 30-2020: A 54-Year-Old Man with Sudden Cardiac Arrest
Elazer R Edelman et al. N Engl J Med. 2020.
No abstract available
Figures
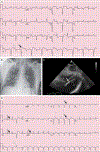
Figure 1.
Initial ECGs, CT Scan of the Head, and Cardiac Catheterization Studies. Electrocardiograms (ECGs) obtained on presentation (Panel A) and after the return of spontaneous circulation (Panel B) show uncertain atrial activity; a regular accelerated idioventricular rhythm at a rate of 70 beats per minute, with a very wide QRS complex and multifocal premature ventricular contractions (white arrows); possible elevations of the ST segment in leads aVR, aVL, and V1; and the presence of U waves (black arrows). An axial computed tomographic (CT) image of the head (Panel C), obtained without the administration of contrast material, was normal, without evidence of infarct, intracranial hemorrhage, or mass lesions. Images from a left coronary angiogram (Panel D) and a right coronary angiogram (Panel E) show only minimal luminal irregularities.
Figure 2.
Additional Cardiac and Imaging Studies. An ECG obtained on the patient’s arrival at the cardiac intensive care unit (ICU) (Panel A) shows sinus tachycardia with alternating left anterior fascicular block (white arrow) and left bundle-branch block (black arrow), as well as corrected QT (QTc) prolongation. A portable chest radiograph (Panel B) shows appropriate positioning of the endotracheal tube, the pulmonary-artery catheter, and the percutaneous ventricular assist device. The lungs are clear, and the cardiac silhouette is normal in size. A transthoracic echocardiogram (subcostal view) (Panel C) shows normal left ventricular size (asterisk) but biventricular dysfunction. The plus sign denotes the right ventricle. The percutaneous ventricular assist device (white arrow) is in an appropriate position, and there is no evidence of pericardial effusion. A repeat ECG obtained in the cardiac ICU (Panel D) shows sinus tachycardia with a normal QRS duration, ventricular ectopic beat (white arrow), nonspecific ST-segment and T-wave abnormalities, U waves (black arrows), and QTc prolongation.
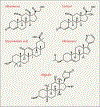
Figure 3.
Chemical Similarities of Drugs That Can Induce Apparent Mineralocorticoid Excess. The notable aspect of each of the increasing number of compounds that can induce apparent mineralocorticoid excess (cortisol, digitalis, glycyrrhetinic acid, and abiraterone) is that they are chemically similar to one another and to aldosterone. Depicted here are the aglycone components of these compounds, which are all congeners of the 21-carbon sterol. Thus, it is not difficult to imagine that each of these compounds could create an acquired apparent mineralocorticoid excess by binding to and activating the mineralocorticoid receptor and other critical receptors in the pathway. In fact, this chemical similarity could well be used to predict the potential of emerging compounds to induce apparent mineralocorticoid excess. R1 and R2 on the digitalis structure reflect the substitution groups that distinguish individual cardiac glycosides.
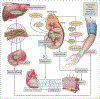
Figure 4.
Physiological Effects of Glycyrrhetinic Acid. Glycyrrhetinic acid was first surmised to have a direct effect on the mineralocorticoid receptor but was subsequently found to have an indirect effect through inhibition of 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2). The resultant unimpeded presence of cortisol can induce hypertension, hypokalemia, metabolic alkalosis, fatal arrhythmias, and renal failure — the constellation of effects seen in this patient. Water and sodium retention and potassium wasting arise from toxic effects of ATPase activity in the collecting duct, further increasing blood pressure through expansion of blood volume. Counterregulatory measures reduce renal renin and angiotensin II secretion and aldosterone production in the adrenal cortex. However, increasing cortisol levels and unrestricted activation of the mineralocorticoid receptor can raise the level of aldosterone (pseudohyperaldosteronism), eliciting a cascade of further elevations in blood volume and cardiac preload. Glycyrrhetinic acid can also inhibit metabolism of aldosterone by hepatic enzymes (5β-reductase and 3β-hydroxysteroid dehydrogenase), thereby further increasing circulating aldosterone levels. Elevated vasomotor tone is maintained as nitric oxide (NO) bioavailability is reduced and vascular endothelin (ET-1) is increased, enhancing contractile responses to pressor hormones. This figure is adapted from Deutch et al. ACE denotes angiotensin-converting enzyme.
Similar articles
- [The old man and the syrup].
Delacour H, Le Berre JP, Servonnet A, Janvier F, Rault A, Ceppa F, Gardet V. Delacour H, et al. Pathol Biol (Paris). 2011 Dec;59(6):336-8. doi: 10.1016/j.patbio.2009.05.008. Epub 2009 Nov 5. Pathol Biol (Paris). 2011. PMID: 19896293 French. - [Severe hypokalemia after holidays return].
Brasseur A, Ducobu J. Brasseur A, et al. Rev Med Brux. 2008 Sep-Oct;29(5):490-3. Rev Med Brux. 2008. PMID: 19055123 French. - [Licorice, aldosterone and blood pressure].
Sane T. Sane T. Duodecim. 1994;110(10):974-80. Duodecim. 1994. PMID: 7588106 Review. Finnish. No abstract available. - [Pseudoaldosteronism].
Kojima K. Kojima K. Ryoikibetsu Shokogun Shirizu. 1993;(1):435-7. Ryoikibetsu Shokogun Shirizu. 1993. PMID: 7757632 Review. Japanese. No abstract available.
Cited by
- Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by Inhibiting the Viral Main Protease.
van de Sand L, Bormann M, Alt M, Schipper L, Heilingloh CS, Steinmann E, Todt D, Dittmer U, Elsner C, Witzke O, Krawczyk A. van de Sand L, et al. Viruses. 2021 Apr 2;13(4):609. doi: 10.3390/v13040609. Viruses. 2021. PMID: 33918301 Free PMC article. - A life-threatening case of pseudo-aldosteronism secondary to excessive liquorice ingestion.
McHugh J, Nagabathula R, Kyithar MP. McHugh J, et al. BMC Endocr Disord. 2021 Aug 6;21(1):158. doi: 10.1186/s12902-021-00816-4. BMC Endocr Disord. 2021. PMID: 34362360 Free PMC article. - Hypertension and Severe Hypokalemia Associated With Oral Ingestion of Topical Hydrocortisone Cream.
Saha A, Balakrishnan S, Trivedi N, Abraham GM. Saha A, et al. AACE Clin Case Rep. 2022 Oct 21;9(1):2-4. doi: 10.1016/j.aace.2022.10.004. eCollection 2023 Jan-Feb. AACE Clin Case Rep. 2022. PMID: 36654996 Free PMC article. - Aldosterone: Renal Action and Physiological Effects.
Johnston JG, Welch AK, Cain BD, Sayeski PP, Gumz ML, Wingo CS. Johnston JG, et al. Compr Physiol. 2023 Mar 30;13(2):4409-4491. doi: 10.1002/cphy.c190043. Compr Physiol. 2023. PMID: 36994769 Free PMC article. - Liquorice Toxicity: A Comprehensive Narrative Review.
Ceccuzzi G, Rapino A, Perna B, Costanzini A, Farinelli A, Fiorica I, Marziani B, Cianci A, Rossin F, Cesaro AE, Spampinato MD, De Giorgio R, Guarino M. Ceccuzzi G, et al. Nutrients. 2023 Sep 5;15(18):3866. doi: 10.3390/nu15183866. Nutrients. 2023. PMID: 37764649 Free PMC article. Review.
References
- Revers FE. Heeft succus liquiritiae een genezende werking op ce maagzweer? Ned Tijdschr Geneeskd 1946;90:135–137. - PubMed
- Revers FE. De behandling van ulcus ventriculi en ulcus duodeni met succus liquiritiae. Ned Tijdschr Geneeskd 1948;92:2968–2973. - PubMed
- Molhuysen JA, Gerbrandy J, de Vries LA, et al. A liquorice extract with deoxycortone-like action. Lancet 1950;2:381–386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources