PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers - PubMed (original) (raw)
Review
PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers
Rui Liu et al. Cell Death Dis. 2020.
Abstract
Multidrug resistance (MDR) is the dominant challenge in the failure of chemotherapy in cancers. Phosphatidylinositol 3-kinase (PI3K) is a lipid kinase that spreads intracellular signal cascades and regulates a variety of cellular processes. PI3Ks are considered significant causes of chemoresistance in cancer therapy. Protein kinase B (AKT) is also a significant downstream effecter of PI3K signaling, and it modulates several pathways, including inhibition of apoptosis, stimulation of cell growth, and modulation of cellular metabolism. This review highlights the aberrant activation of PI3K/AKT as a key link that modulates MDR. We summarize the regulation of numerous major targets correlated with the PI3K/AKT pathway, which is further related to MDR, including the expression of apoptosis-related protein, ABC transport and glycogen synthase kinase-3 beta (GSK-3β), synergism with nuclear factor kappa beta (NF-κB) and mammalian target of rapamycin (mTOR), and the regulation of glycolysis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
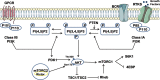
Fig. 1. As a major downstream effector of receptor tyrosine kinase (RTK) and G protein-coupled receptors, PI3K activates various downstream effectors by generating phospholipids, transducing signals of various growth factors and cytokines into intracellular information.
The main lipid substrate of PTEN is PIP 3 and indeed PTEN acts as a negative regulator of PI3K/AKT signaling. Among the upstream signaling networks, Akt inactivate TSC1/2 and activate mTORC1. mTORC2 directly phosphorylates Akt at S473 residue leading to its complete activation. This activation of the PI3K/Akt pathway is opposed by PTEN.
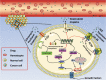
Fig. 2. The PI3K/AKT pathway regulates the gene expression of ABC transporters by activating downstream targets, such as NF-κB and Nfr2, resulting in over-expression of P-gp, MRP1, BCRP.
Thus, accelerating the transport of anticancer drugs by ABC transporter to the outside that leading to MDR.
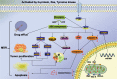
Fig. 3. A dysfunctional PI3K/AKT pathway serves as a hub to regulate many cellular processes that are involved in MDR including apoptosis, ABC transporter activity, mTOR pathway, and tumor metabolism.
Note: The PI3K/AKT pathway enhances drug efflux through effective expression of ABC transporter. Aerobic glycolysis is the key energy supplier to the metabolic adaptation of cancer cells for MDR. The PI3K/AKT pathway affects tumor proliferation by regulating mTOR, GSK-3β, and NF-κB. The PI3K/AKT pathway triggers XIAP to suppress the activity of caspase-3 inhibiting apoptosis. The decrease of caspase-8 will reduce migration of Bid to mitochondria, and slim down the activation of Bax and Bim, which lead to the decrease of mitochondrial membrane permeability (MOMP) and the release of cytochrome c. Caspase-8 also regulates the expression of caspase-3 to achieve cell apoptosis. The P13K/AKT/mTOR may also underlie MDR in tumor cells through inducing dysregulation of miRNA.
Similar articles
- Role of PI3K/Akt/NF-κB and GSK-3β pathways in the rat model of cardiopulmonary bypass-related lung injury.
He M, Zhang Y, Xie F, Dou X, Han M, Zhang H. He M, et al. Biomed Pharmacother. 2018 Oct;106:747-754. doi: 10.1016/j.biopha.2018.06.125. Epub 2018 Jul 11. Biomed Pharmacother. 2018. PMID: 29990867 - Anti-proliferative effect of RCE-4 from Reineckia carnea on human cervical cancer HeLa cells by inhibiting the PI3K/Akt/mTOR signaling pathway and NF-κB activation.
Bai C, Yang X, Zou K, He H, Wang J, Qin H, Yu X, Liu C, Zheng J, Cheng F, Chen J. Bai C, et al. Naunyn Schmiedebergs Arch Pharmacol. 2016 Jun;389(6):573-84. doi: 10.1007/s00210-016-1217-7. Epub 2016 Mar 3. Naunyn Schmiedebergs Arch Pharmacol. 2016. PMID: 26935715 - Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway.
Polivka J Jr, Janku F. Polivka J Jr, et al. Pharmacol Ther. 2014 May;142(2):164-75. doi: 10.1016/j.pharmthera.2013.12.004. Epub 2013 Dec 9. Pharmacol Ther. 2014. PMID: 24333502 Review. - Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside.
Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM, Manzoli L, McCubrey JA, Cocco L. Martelli AM, et al. Curr Med Chem. 2007;14(19):2009-23. doi: 10.2174/092986707781368423. Curr Med Chem. 2007. PMID: 17691943 Review.
Cited by
- Synergistic effects of curcumin and stem cells on spinal cord injury: a comprehensive review.
Arefnezhad R, Jahandideh A, Rezaei M, Khatouni MS, Zarei H, Jahani S, Molavi A, Hefzosseheh M, Ghasempour P, Movahedi HM, Jahandideh R, Rezaei-Tazangi F. Arefnezhad R, et al. Mol Biol Rep. 2024 Nov 1;51(1):1113. doi: 10.1007/s11033-024-10057-y. Mol Biol Rep. 2024. PMID: 39485550 Review. - The Role of the PI3K/Akt/mTOR Axis in Head and Neck Squamous Cell Carcinoma.
Jiang Q, Xiao J, Hsieh YC, Kumar NL, Han L, Zou Y, Li H. Jiang Q, et al. Biomedicines. 2024 Jul 19;12(7):1610. doi: 10.3390/biomedicines12071610. Biomedicines. 2024. PMID: 39062182 Free PMC article. Review. - Reregulated mitochondrial dysfunction reverses cisplatin resistance microenvironment in colorectal cancer.
Wang Y, Ma X, Zhou W, Liu C, Zhang H. Wang Y, et al. Smart Med. 2022 Dec 22;1(1):e20220013. doi: 10.1002/SMMD.20220013. eCollection 2022 Dec. Smart Med. 2022. PMID: 39188744 Free PMC article. - MicroRNAs targeted mTOR as therapeutic agents to improve radiotherapy outcome.
Taeb S, Rostamzadeh D, Amini SM, Rahmati M, Eftekhari M, Safari A, Najafi M. Taeb S, et al. Cancer Cell Int. 2024 Jul 4;24(1):233. doi: 10.1186/s12935-024-03420-3. Cancer Cell Int. 2024. PMID: 38965615 Free PMC article. Review. - Evidence of the Beneficial Effects of Ursolic Acid against Lung Cancer.
Kornel A, Nadile M, Tsiani E. Kornel A, et al. Molecules. 2022 Nov 2;27(21):7466. doi: 10.3390/molecules27217466. Molecules. 2022. PMID: 36364289 Free PMC article. Review.
References
- Sharma A. Chemoresistance in cancer cells: exosomes as potential regulators of therapeutic tumor heterogeneity. Nanomedicine. 2017;12:2137–2148. - PubMed
- Garcia-Mayea Y, Mir C, Masson F, Paciucci R, LLeonart ME. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020;60:166–180. - PubMed
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2:48–58. - PubMed
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous