Mitochondria Targeted Viral Replication and Survival Strategies-Prospective on SARS-CoV-2 - PubMed (original) (raw)
Review
Mitochondria Targeted Viral Replication and Survival Strategies-Prospective on SARS-CoV-2
Priya Gatti et al. Front Pharmacol. 2020.
Abstract
SARS-CoV-2 is a positive sense RNA coronavirus that constitutes a new threat for the global community and economy. While vaccines against SARS-CoV-2 are being developed, the mechanisms through which this virus takes control of an infected cell to replicate remains poorly understood. Upon infection, viruses completely rely on host cell molecular machinery to survive and replicate. To escape from the immune response and proliferate, viruses strategically modulate cellular metabolism and alter subcellular organelle architecture and functions. One way they do this is by modulating the structure and function of mitochondria, a critical cellular metabolic hub but also a key platform for the regulation of cellular immunity. This versatile nature of mitochondria defends host cells from viruses through several mechanisms including cellular apoptosis, ROS signaling, MAVS activation and mitochondrial DNA-dependent immune activation. These events are regulated by mitochondrial dynamics, a process by which mitochondria alter their structure (including their length and connectivity) in response to stress or other cues. It is therefore not surprising that viruses, including coronaviruses hijack these processes for their survival. In this review, we highlight how positive sense RNA viruses modulate mitochondrial dynamics and metabolism to evade mitochondrial mediated immune response in order to proliferate.
Keywords: RNA viruses; SARS-CoV-2; immune response; metabolism; mitochondria; mitochondrial dynamics.
Copyright © 2020 Gatti, Ilamathi, Todkar and Germain.
Figures
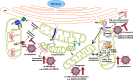
Figure 1
Effects of (+)ssRNA viruses on mitochondrial dynamics and function. By manipulating mitochondrial dynamics (1), (+)ssRNA viruses either directly or indirectly manipulate a number of cellular processes to promote viral replication and immune evasion. These include increasing mitochondrial activity (2), inhibiting MAVS activation (3) and activating mitophagy (4) to prevent apoptosis (5). In the case of SARS-CoV (and probably SARS-CoV-2), the ORF9b protein can perform most of these functions.
Similar articles
- Severe acute respiratory syndrome coronaviruses contributing to mitochondrial dysfunction: Implications for post-COVID complications.
Prasada Kabekkodu S, Chakrabarty S, Jayaram P, Mallya S, Thangaraj K, Singh KK, Satyamoorthy K. Prasada Kabekkodu S, et al. Mitochondrion. 2023 Mar;69:43-56. doi: 10.1016/j.mito.2023.01.005. Epub 2023 Jan 20. Mitochondrion. 2023. PMID: 36690315 Free PMC article. Review. - The double-membrane vesicle (DMV): a virus-induced organelle dedicated to the replication of SARS-CoV-2 and other positive-sense single-stranded RNA viruses.
Roingeard P, Eymieux S, Burlaud-Gaillard J, Hourioux C, Patient R, Blanchard E. Roingeard P, et al. Cell Mol Life Sci. 2022 Jul 16;79(8):425. doi: 10.1007/s00018-022-04469-x. Cell Mol Life Sci. 2022. PMID: 35841484 Free PMC article. Review. - SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome.
Shi CS, Qi HY, Boularan C, Huang NN, Abu-Asab M, Shelhamer JH, Kehrl JH. Shi CS, et al. J Immunol. 2014 Sep 15;193(6):3080-9. doi: 10.4049/jimmunol.1303196. Epub 2014 Aug 18. J Immunol. 2014. PMID: 25135833 Free PMC article. - Pathophysiological involvement of host mitochondria in SARS-CoV-2 infection that causes COVID-19: a comprehensive evidential insight.
Bhowal C, Ghosh S, Ghatak D, De R. Bhowal C, et al. Mol Cell Biochem. 2023 Jun;478(6):1325-1343. doi: 10.1007/s11010-022-04593-z. Epub 2022 Oct 29. Mol Cell Biochem. 2023. PMID: 36308668 Free PMC article. Review. - Role of Mitochondria in Viral Infections.
Elesela S, Lukacs NW. Elesela S, et al. Life (Basel). 2021 Mar 11;11(3):232. doi: 10.3390/life11030232. Life (Basel). 2021. PMID: 33799853 Free PMC article. Review.
Cited by
- Nanobody against SARS-CoV-2 non-structural protein Nsp9 inhibits viral replication in human airway epithelia.
Venit T, Blavier J, Maseko SB, Shu S, Espada L, Breunig C, Holthoff HP, Desbordes SC, Lohse M, Esposito G, Twizere JC, Percipalle P. Venit T, et al. Mol Ther Nucleic Acids. 2024 Aug 15;35(3):102304. doi: 10.1016/j.omtn.2024.102304. eCollection 2024 Sep 10. Mol Ther Nucleic Acids. 2024. PMID: 39281707 Free PMC article. - The mitochondrial UPR regulator ATF5 promotes intestinal barrier function via control of the satiety response.
Chamseddine D, Mahmud SA, Westfall AK, Castoe TA, Berg RE, Pellegrino MW. Chamseddine D, et al. Cell Rep. 2022 Dec 13;41(11):111789. doi: 10.1016/j.celrep.2022.111789. Cell Rep. 2022. PMID: 36516750 Free PMC article. - MAVS: A Two-Sided CARD Mediating Antiviral Innate Immune Signaling and Regulating Immune Homeostasis.
Chen Y, Shi Y, Wu J, Qi N. Chen Y, et al. Front Microbiol. 2021 Sep 9;12:744348. doi: 10.3389/fmicb.2021.744348. eCollection 2021. Front Microbiol. 2021. PMID: 34566944 Free PMC article. Review. - SARS-CoV-2 infection and replication kinetics in different human cell types: The role of autophagy, cellular metabolism and ACE2 expression.
Bartolomeo CS, Lemes RMR, Morais RL, Pereria GC, Nunes TA, Costa AJ, de Barros Maciel RM, Braconi CT, Maricato JT, Janini LMR, Okuda LH, Lee KS, Prado CM, Ureshino RP, Stilhano RS. Bartolomeo CS, et al. Life Sci. 2022 Nov 1;308:120930. doi: 10.1016/j.lfs.2022.120930. Epub 2022 Sep 6. Life Sci. 2022. PMID: 36075471 Free PMC article. - COVID-19 metabolism: Mechanisms and therapeutic targets.
Wang T, Cao Y, Zhang H, Wang Z, Man CH, Yang Y, Chen L, Xu S, Yan X, Zheng Q, Wang YP. Wang T, et al. MedComm (2020). 2022 Aug 9;3(3):e157. doi: 10.1002/mco2.157. eCollection 2022 Sep. MedComm (2020). 2022. PMID: 35958432 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous