Uridine diphosphate N-acetylglucosamine orchestrates the interaction of GlmR with either YvcJ or GlmS in Bacillus subtilis - PubMed (original) (raw)
Uridine diphosphate N-acetylglucosamine orchestrates the interaction of GlmR with either YvcJ or GlmS in Bacillus subtilis
Elodie Foulquier et al. Sci Rep. 2020.
Abstract
In bacteria, glucosamine-6-phosphate (GlcN6P) synthase, GlmS, is an enzyme required for the synthesis of Uridine diphosphate N-acetylglucosamine (UDP-GlcNAc), a precursor of peptidoglycan. In Bacillus subtilis, an UDP-GlcNAc binding protein, GlmR (formerly YvcK), essential for growth on non-glycolytic carbon sources, has been proposed to stimulate GlmS activity; this activation could be antagonized by UDP-GlcNAc. Using purified proteins, we demonstrate that GlmR directly stimulates GlmS activity and the presence of UDP-GlcNAc (at concentrations above 0.1 mM) prevents this regulation. We also showed that YvcJ, whose gene is associated with yvcK (glmR), interacts with GlmR in an UDP-GlcNAc dependent manner. Strains producing GlmR variants unable to interact with YvcJ show decreased transformation efficiency similar to that of a yvcJ null mutant. We therefore propose that, depending on the intracellular concentration of UDP-GlcNAc, GlmR interacts with either YvcJ or GlmS. When UDP-GlcNAc concentration is high, this UDP-sugar binds to YvcJ and to GlmR, blocking the stimulation of GlmS activity and driving the interaction between GlmR and YvcJ to probably regulate the cellular role of the latter. When the UDP-GlcNAc level is low, GlmR does not interact with YvcJ and thus does not regulate its cellular role but interacts with GlmS to stimulate its activity.
Conflict of interest statement
The authors declare no competing interests.
Figures
Figure 1
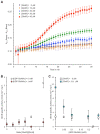
Measurement of GlmS activity in the presence or in the absence of GlmR and UDP-GlcNAc. Activity of GlmS from B. subtilis was measured by enzyme coupled assay. For each experiment, a reaction without GlmS was performed as negative control and used for background correction. Each experiment was reproduced at least in triplicate and error bars represent standard deviations. (A) Kinetic of GlmS activity in the presence of increasing concentration of GlmR. 7.3 µM of GlmS (48 µg in 100 µl) were incubated in the presence 0, 10, 20, 30 and 40 µM of GlmR in a final volume of 100 µl as indicated in the experimental procedures section. The amount of CoASH produced was monitored at 412 nm during 30 min by microplate reader at 37 °C as described previously. We observed that the effect of GlmR on GlmS activity is optimal after 8 min of incubation; consequently, to calculate the amount of GlcN6P produced per min by GlmS, we measured the slope between 18 and 8 min. (B) GlmS activity in the presence of increasing amount of GlmR in the absence or in the presence of 1 mM UDP-GlcNAc. 7.3 µM of GlmS (48 µg in 100 µl) were incubated in the presence 0, 5.4, 10.7, 16.1, 21.4 and 32.1 µM of GlmR as indicated in the material and methods section. The amount of CoASH produced was monitored at 412 nm by microplate reader at 37 °C and the specific activity of GlmS was calculated as indicated in Figs. S1 and S2. (C) GlmS activity in the presence of increasing amount of UDP-GlcNAc and in the absence or in the presence of GlmR. 5.3 µM of GlmS (35 µg in 100 µl) were incubated in the absence or in the presence of 32.1 µM of GlmR ([GlmR]/[GlmS] = 6) and 0, 0.05, 0.1, 0.2, 0.5 and 1 mM of UDP-GlcNAc. The amount of CoASH produced was monitored at 412 nm by microplate reader at 37 °C and the specific activity of GlmS was calculated as indicated previously.
Figure 2
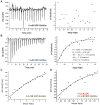
Genomic context of glmR (yvcK) and analysis of a potential interaction between GlmR and YvcJ by bacterial two hybrid. (A) Genomic context of glmR using Version 9.0 of STRING (
). Concerning Archaea, the gene homologous to glmR encodes CofD, a 2-phospho-lactate transferase that catalyzes the last step in the biosynthesis of coenzyme F(420). CofD is highly conserved among F(420)-producing organisms but possesses weak sequence homology with GlmR (or YvcK) found in non-F(420)-producing organisms. In these non-F(420)-producing bacteria, yvcK (glmR) seems always associated to yvcJ (or rapZ). (B) Analysis of GlmR_,_ GlmR(R301A), GlmR(Y265A) interactions with YvcJ by bacterial two hybrid assays. The T18 and T25 fragments of the adenylate cyclase protein were fused to GlmR_,_ GlmR(R301A), GlmR(Y265A_)_ and YvcJ. The pT18 and pT25 derivative plasmids were transformed into E. coli strain BTH101 that were spotted onto LB medium supplemented with X-Gal and IPTG and incubated at 30 °C overnight. When co-produced protein fusions interact, the Bordetella pertussis adenylate cyclase is active and the colonies are blue in the presence of X-Gal. Cells containing pT18 and pT25 empty vectors were used as negative control and cells containing pT18-ZIP and pT25-ZIP plasmids as positive control. Each experiment was reproduced at least in triplicate. Full-length picture of the petri dish is presented in Fig. S6.
Figure 3
Analysis of the interaction of GlmR with YvcJ by ITC. For all the experiments, the reference experiment with the titrant protein injected into the cell containing buffer 50 mM Tris–HCl pH 7.5, 50 mM NaCl and 5% glycerol and 0, 0.4 or 1 mM UDP-GlcNAc was subtracted from the experimental data before analysis. Each experiment was reproduced at least in triplicate. (A) YvcJ in the absence of UDP-GlcNAc. (B) YvcJ in the presence of 1 mM UDP-GlcNAc. For these two experiments, the titrant protein (in the syringe) is GlmR at a concentration of 210 µM; it is injected into the sample cell containing 16 µM of YvcJ. The left panel shows heat exchange upon ligand titration and right panel shows the corresponding integrated data with binding isotherms fitted to a single–site binding model. (C) YvcJ in the presence of 0.4 mM UDP-GlcNAc. The titrant YvcJ (88 µM) was injected into a cell containing 2.5 µM GlmR at 37 °C in the presence of 0.4 mM UDP-GlcNAc. (D) YvcJ(K22A) in the presence of 0.4 mM UDP-GlcNAc. The titrant YvcJ(K22A) (109 µM) was injected into a cell containing 10 µM GlmR at 37 °C in the presence of 0.4 mM UDP-GlcNAc. For these two experiments, only the integrated data with binding isotherms fitted to a single–site binding model are presented. However, the interaction between YvcJ (WT or K22A) and GlmR is too weak to determine the thermodynamic parameters and the _K_D in a reliable and accurate way.
Figure 4
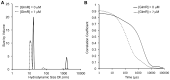
Analysis of the effect of GlmR on YvcJ by dynamic light scattering (DLS). (A) Volume weighted size distributions of 24 µM YvcJ in the presence of 0.4 mM UDP-GlcNAc and in the absence or in the presence of 7 µM GlmR at 25 °C using DLS. In black YvcJ is alone with the UDP-sugar. In black dashed line GlmR is added to YvcJ with a ratio of 1:0.3 YvcJ:GlmR. Three independent measurements were performed for each sample. (B) Real time correlation of intensity over time of YvcJ and GlmR in the presence of 0.4 mM UDP-GlcNAc average of triplicate. In black YvcJ is alone with the UDP-sugar and in black dashed GlmR is added to YvcJ with a ratio of 1:0.3 YvcJ:GlmR.
Figure 5
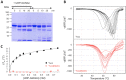
Investigation of the potential binding of UDP-GlcNAc to YvcJ by partial proteolysis and Thermal Shift Assay (TSA). (A) Coomassie-stained SDS-PAGE of YvcJ partial proteolysis profile. YvcJ was incubated with endoproteinase Glu-C (Promega) in the presence or in the absence of 1 mM UDP-GlcNAc for 0, 5, 10 or 20 min at 37 °C. The digestion profiles were assessed by electrophoresis in 12.5% SDS-PAGE. Full-length gel is presented in Fig. S8. (B) TSA in the presence of increasing concentrations of UDP-GlcNAc. YvcJ (top) and YvcJ(K22A) (bottom) melting profiles were monitored in the presence of increasing concentration of UDP-GlcNAc (0–1 mM). One curve corresponds to data obtained for one concentration of UDP-GlcNAc. The melting temperature of the protein (Tm) is obtained at the midpoint of each melting curve and corresponds to the minimum of the negative derivative curves. The Tm is an indicator of protein stability and is increased by the addition of UDP-GlcNAc. For the WT protein, Tm = 48 °C in the absence of UDP-GlcNAc and Tm = 58.5 °C in the presence of 1 mM UDP-GlcNAc. For YvcJ(K22A), Tm = 49.5 °C and is not increased by addition of UDP-GlcNAc. (C) TSA results for the binding of UDP-GlcNAc to YvcJ. Assays were performed with YvcJ and YvcJ(K22A) in the presence of increasing concentrations of ligands (0–0.7 mM). The difference of temperature (the shift of Tm induced by the presence of ligand) was plotted against the concentration of UDP-GlcNAc. Each experiment was reproduced at least in triplicate and the standard deviations are represented by the error bars. Curve fitting was performed by using Microcal Origin 5.0 software (Microcal software Inc).
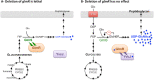
Figure 6
Schematic representation of GlmS regulation by GlmR, UDP-GlcNAc and YvcJ in B. subtilis depending on growth conditions. (A) When B. subtilis is grown in the presence of non-glycolytic carbon sources like intermediates of Krebs cycle, the glmR mutant cells have an abnormal rod-shape then lyse and the deletion of glmR is lethal. In such conditions, stimulation of GlmS by GlmR is essential for a sufficient production of UDP-GlcNAc and thus for a correct PG synthesis; YvcJ is free. (B) When B. subtilis is grown in the presence of glycolytic carbon sources like glucose, the glmR mutant cells have a normal rod-shape and deletion of glmR has no effect. The intracellular concentration of F6P is high, 16-fold higher in comparison to growth on malate. Consequently, intracellular concentration of UDP-GlcNAc is probably high and therefore stimulation of GlmS by GlmR is not essential for correct synthesis of PG. In such conditions, GlmR is bound to UDP-GlcNAc and GlmS activity is not stimulated to avoid an excess of UDP-GlcNAc synthesis. In addition, GlmR bound to UDP-GlcNAc interacts with YvcJ to stabilize it and potentially stimulate its activity. In parallel, GlcN6P binds to glmS ribozyme and regulates GlmS intracellular concentration.
Similar articles
- Reciprocal regulation of enterococcal cephalosporin resistance by products of the autoregulated yvcJ-glmR-yvcL operon enhances fitness during cephalosporin exposure.
Djorić D, Atkinson SN, Kristich CJ. Djorić D, et al. PLoS Genet. 2024 Mar 21;20(3):e1011215. doi: 10.1371/journal.pgen.1011215. eCollection 2024 Mar. PLoS Genet. 2024. PMID: 38512984 Free PMC article. - A metabolic checkpoint protein GlmR is important for diverting carbon into peptidoglycan biosynthesis in Bacillus subtilis.
Patel V, Wu Q, Chandrangsu P, Helmann JD. Patel V, et al. PLoS Genet. 2018 Sep 24;14(9):e1007689. doi: 10.1371/journal.pgen.1007689. eCollection 2018 Sep. PLoS Genet. 2018. PMID: 30248093 Free PMC article. - Listeria monocytogenes GlmR Is an Accessory Uridyltransferase Essential for Cytosolic Survival and Virulence.
Pensinger DA, Gutierrez KV, Smith HB, Vincent WJB, Stevenson DS, Black KA, Perez-Medina KM, Dillard JP, Rhee KY, Amador-Noguez D, Huynh TN, Sauer JD. Pensinger DA, et al. mBio. 2023 Apr 25;14(2):e0007323. doi: 10.1128/mbio.00073-23. Epub 2023 Mar 20. mBio. 2023. PMID: 36939339 Free PMC article. - Highlights of glucosamine-6P synthase catalysis.
Durand P, Golinelli-Pimpaneau B, Mouilleron S, Badet B, Badet-Denisot MA. Durand P, et al. Arch Biochem Biophys. 2008 Jun 15;474(2):302-17. doi: 10.1016/j.abb.2008.01.026. Epub 2008 Feb 6. Arch Biochem Biophys. 2008. PMID: 18279655 Review. - Enzymes of UDP-GlcNAc biosynthesis in yeast.
Milewski S, Gabriel I, Olchowy J. Milewski S, et al. Yeast. 2006 Jan 15;23(1):1-14. doi: 10.1002/yea.1337. Yeast. 2006. PMID: 16408321 Review.
Cited by
- Adaptation of Bacillus subtilis MreB Filaments to Osmotic Stress Depends on Influx of Potassium Ions.
Dersch S, Graumann PL. Dersch S, et al. Microorganisms. 2024 Jun 27;12(7):1309. doi: 10.3390/microorganisms12071309. Microorganisms. 2024. PMID: 39065078 Free PMC article. - Preliminary X-ray diffraction and ligand-binding analyses of the N-terminal domain of hypothetical protein Rv1421 from Mycobacterium tuberculosis H37Rv.
Park J, Cheon YJ, Jeong YC, Lee KS. Park J, et al. Acta Crystallogr F Struct Biol Commun. 2024 Jul 1;80(Pt 7):135-141. doi: 10.1107/S2053230X24005831. Epub 2024 Jun 27. Acta Crystallogr F Struct Biol Commun. 2024. PMID: 38935514 Free PMC article. - Reciprocal regulation of enterococcal cephalosporin resistance by products of the autoregulated yvcJ-glmR-yvcL operon enhances fitness during cephalosporin exposure.
Djorić D, Atkinson SN, Kristich CJ. Djorić D, et al. PLoS Genet. 2024 Mar 21;20(3):e1011215. doi: 10.1371/journal.pgen.1011215. eCollection 2024 Mar. PLoS Genet. 2024. PMID: 38512984 Free PMC article. - On the mechanisms of lysis triggered by perturbations of bacterial cell wall biosynthesis.
Kawai Y, Kawai M, Mackenzie ES, Dashti Y, Kepplinger B, Waldron KJ, Errington J. Kawai Y, et al. Nat Commun. 2023 Jul 11;14(1):4123. doi: 10.1038/s41467-023-39723-8. Nat Commun. 2023. PMID: 37433811 Free PMC article. - Recent Advances in Peptidoglycan Synthesis and Regulation in Bacteria.
Galinier A, Delan-Forino C, Foulquier E, Lakhal H, Pompeo F. Galinier A, et al. Biomolecules. 2023 Apr 22;13(5):720. doi: 10.3390/biom13050720. Biomolecules. 2023. PMID: 37238589 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases