Alignment of Alzheimer's disease amyloid β-peptide and klotho - PubMed (original) (raw)
Alignment of Alzheimer's disease amyloid β-peptide and klotho
Steven Lehrer et al. World Acad Sci J. 2020 Nov-Dec.
Abstract
The cause of Alzheimer's disease (AD) is poorly understood. In 1991, the amyloid hypothesis postulated that β-amyloid (Aβ) accumulation is a key element. It follows that clearing the brain of Aβ would be beneficial, which has not been the case. Therefore, Aβ is likely a result, not a cause, of AD and may be protective rather than harmful. The apolipoprotein E4 (apoE4) allele is the strongest genetic risk factor for AD. Klotho (KL), encoded by the KL gene, may be another AD-related protein. FGF21 is a circulating endocrine hormone, mainly secreted by the liver, mostly during fasting. FGF21 acts by binding to its receptor FGFR1 and co-receptor β-klotho. FGF21 is neuroprotective and could delay onset of AD. In the present study, the KL protein structure was examined to determine whether it may interact with Aβ. Protein data bank (pdb) entries for klotho and Aβ were searched on the RCSB Protein Data Bank for β-KL and AD amyloid β-peptide. The protein structures were superimposed and aligned on PYMOL v2.3.4 with the super command, which super aligns two protein selections. To evaluate the conservation and alignment of the Aβ and KL genomes across species, BLAT, the Blast-Like Alignment Tool of the UCSC Genome Browser, was used. The amino acid residues phe76-val96 of KL aligned closely with residues asp7-asn27 of Aβ. Cross-species comparison of KL revealed a high degree of alignment and conservation in the chimp and 27 other primates; however, less alignment and conservation were observed in the mouse, dog and elephant, even less in the chicken, western clawed frog (Xenopus tropicalis), zebrafish and lamprey. The current finding of amino acid residues phe76-val96 of klotho aligning closely with residues asp7-asn27 of Aβ suggests that Aβ can enhance the ability of klotho to draw FGF21 to regions of incipient neurodegeneration in AD. The problem arises with age. Older individuals do not heal or repair tissue damage as well as younger individuals. As neurodegeneration advances in an older individual, perhaps caused by neuroinflammation related to herpes simplex virus type 1, increasing amounts of amyloid are produced, forming an adhesive web, as the brain tries to hold the pathologic process in check. Meanwhile, the damage increases and spreads. Progressive neurodegeneration and cognitive decline are the outcome.
Keywords: Alzheimer’s disease; HSV-1; aging; alignment; klotho; neurodegeneration; neuroinflammation; protein; ubiquitin; β-amyloid.
Conflict of interest statement
Competing interests The authors declare that they have no competing interests.
Figures
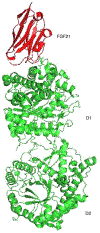
Figure 1.
Crystal structure of β-Klotho showing FGF21 (red) bound to domain 1 (D1).
Figure 2.
Solution structure of the Alzheimer’s disease Aβ-peptide.
Figure 3.
Alignment of klotho peptide (green) domain 1 (D1) with Aβ (blue). FGF21 (red) is bound to domain 1.
Figure 4.
Closeup of alignment. Pymol performed 6 cycles of calculations on 165 aligned atoms, with a final root mean square deviation of atomic positions (RMSD) of 1.792 Å for 148 atoms. Amino acid residues phe76-val96 of klotho aligned closely with residues asp7-asn27 of Aβ.
Figure 5.
Alignment of 42 amino acid residue human Aβ across species in the UCSC genome browser. There is a high degree of alignment and conservation of Aβ (chr 21q21.3) in the rhesus monkey and 27 other primates, but much less alignment and conservation in the mouse, dog, and elephant, even less in the chicken, western clawed frog (Xenopus tropicalis), zebrafish and lamprey.
Figure 6.
Alignment of human klotho across species in the UCSC genome browser. Results of the cross-species comparison of klotho show a high degree of alignment and conservation of human klotho (chr 13q13.1) in the chimp and 27 other primates, less alignment and conservation in the mouse, dog, and elephant, even less in the chicken, western clawed frog (Xenopus tropicalis), zebrafish and lamprey.
Similar articles
- Alignment of Alzheimer's Disease Amyloid-β Peptide and Herpes Simplex Virus-1 pUL15 C-Terminal Nuclease Domain.
Lehrer S, Rheinstein PH. Lehrer S, et al. J Alzheimers Dis Rep. 2020 Sep 9;4(1):373-377. doi: 10.3233/ADR-200231. J Alzheimers Dis Rep. 2020. PMID: 33163898 Free PMC article. - Klotho allele status is not associated with Aβ and APOE ε4-related cognitive decline in preclinical Alzheimer's disease.
Porter T, Burnham SC, Milicic L, Savage G, Maruff P, Lim YY, Ames D, Masters CL, Martins RN, Rainey-Smith S, Rowe CC, Salvado O, Groth D, Verdile G, Villemagne VL, Laws SM. Porter T, et al. Neurobiol Aging. 2019 Apr;76:162-165. doi: 10.1016/j.neurobiolaging.2018.12.014. Epub 2019 Jan 6. Neurobiol Aging. 2019. PMID: 30716541 - Amyloid-β positivity is less prevalent in cognitively unimpaired KLOTHO KL-VS heterozygotes.
Cook N, Driscoll I, Gaitán JM, Glittenberg M, Betthauser TJ, Carlsson CM, Johnson SC, Asthana S, Zetterberg H, Blennow K, Kollmorgen G, Quijano-Rubio C, Dubal DB, Okonkwo OC. Cook N, et al. J Alzheimers Dis. 2024 Nov;102(2):480-490. doi: 10.1177/13872877241289785. Epub 2024 Nov 11. J Alzheimers Dis. 2024. PMID: 39529379 - Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review.
Butterfield DA. Butterfield DA. Free Radic Res. 2002 Dec;36(12):1307-13. doi: 10.1080/1071576021000049890. Free Radic Res. 2002. PMID: 12607822 Review. - Alzheimer's disease.
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV. De-Paula VJ, et al. Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
- Klotho an Autophagy Stimulator as a Potential Therapeutic Target for Alzheimer's Disease: A Review.
Fung TY, Iyaswamy A, Sreenivasmurthy SG, Krishnamoorthi S, Guan XJ, Zhu Z, Su CF, Liu J, Kan Y, Zhang Y, Wong HLX, Li M. Fung TY, et al. Biomedicines. 2022 Mar 18;10(3):705. doi: 10.3390/biomedicines10030705. Biomedicines. 2022. PMID: 35327507 Free PMC article. Review.
References
- Hardy J and Allsop D: Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 12: 383–388, 1991. - PubMed
- Castellani RJ and Perry G: The complexities of the pathology-pathogenesis relationship in Alzheimer disease. Biochem Pharmacol 88: 671–676, 2014. - PubMed
- Moir RD, Lathe R and Tanzi RE: The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimers Dement 14: 1602–1614, 2018. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous