Viral and host heterogeneity and their effects on the viral life cycle - PubMed (original) (raw)
Review
Viral and host heterogeneity and their effects on the viral life cycle
Jennifer E Jones et al. Nat Rev Microbiol. 2021 Apr.
Abstract
Traditionally, the viral replication cycle is envisioned as a single, well-defined loop with four major steps: attachment and entry into a target cell, replication of the viral genome, maturation of viral proteins and genome packaging into infectious progeny, and egress and dissemination to the next target cell. However, for many viruses, a growing body of evidence points towards extreme heterogeneity in each of these steps. In this Review, we reassess the major steps of the viral replication cycle by highlighting recent advances that show considerable variability during viral infection. First, we discuss heterogeneity in entry receptors, followed by a discussion on error-prone and low-fidelity polymerases and their impact on viral diversity. Next, we cover the implications of heterogeneity in genome packaging and assembly on virion morphology. Last, we explore alternative egress mechanisms, including tunnelling nanotubes and host microvesicles. In summary, we discuss the implications of viral phenotypic, morphological and genetic heterogeneity on pathogenesis and medicine. This Review highlights common themes and unique features that give nuance to the viral replication cycle.
Conflict of interest statement
The authors declare no competing interests.
Figures
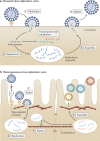
Fig. 1. Heterogeneity in the virus replication cycle.
a | Traditional depiction of the viral replication cycle with its series of defined, uniform steps, using human metapneumovirus (HMPV) as an example. HMPV virions bind to a target receptor and fuse with the host cell membrane (step 1). After fusion, viral RNA is replicated in cytoplasmic replication organelles (step 2) and viral proteins are produced and undergo assembly with the viral RNA (step 3). Homogeneous mature virus particles undergo egress through the cellular membrane (step 4). b | Evidence of substantial heterogeneity in the viral replication cycle is accumulating. Entry can be impacted by the presence of post-translational modification of receptors, including glycosylation (step 1). Upon entry, HMPV replication organelles can traffic along actin-containing nanotubes across tight junctions of the lung epithelium (step 2). Variation in assembly (step 3) and egress (step 4) lead to the maturation of heterogeneous virus particles from the cell. Similar heterogeneity has been observed in many different viruses.
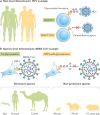
Fig. 2. Heterogeneity in glycosylation of entry receptors.
a | Differences between individual hosts can affect viral entry, such as heterogeneous host populations (left) that carry differentially modified viral receptors. In the example shown, hepatitis C virus (HCV) has different affinities for variants of its co-receptor, scavenger receptor class B type I (SR-BI). The glycosylated wild-type (WT) T175 variant (top) binds HCV, and the unmodified T175A variant found in human SR-BI (bottom) does not. b | Furthermore, species-level determinants of viral entry exist. Middle East respiratory syndrome coronavirus (MERS-CoV) differs in its affinity for different variants of the receptor dipeptidyl peptidase 4 (DPP4). Humans, camels and bats carry unmodified DPP4 (left), which binds MERS-CoV, whereas the glycosylated DPP4 variants found in mice, ferrets and guinea pigs (right) do not support infection. *Additional determinants exist.
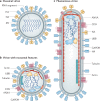
Fig. 3. Heterogeneity in influenza virus particle morphology.
a | Traditionally, all pleomorphic influenza virus particles were thought to comprise viral components only. This traditional influenza virus particle is shown, displaying spherical morphology and consisting of haemagglutinin (HA), neuraminidase (NA), matrix (M1) and viral genomic material. b | Careful studies of virions have revealed a revised architecture consisting of an influenza virion that encases several components of host exosomes. c | Influenza virus also assumes a filamentous morphology of variable length with polarized NA proteins. ANXA, annexin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; UBB, ubiquitin.
Similar articles
- Viroporins customize host cells for efficient viral propagation.
Giorda KM, Hebert DN. Giorda KM, et al. DNA Cell Biol. 2013 Oct;32(10):557-64. doi: 10.1089/dna.2013.2159. Epub 2013 Aug 14. DNA Cell Biol. 2013. PMID: 23945006 Free PMC article. Review. - Opportunistic intruders: how viruses orchestrate ER functions to infect cells.
Ravindran MS, Bagchi P, Cunningham CN, Tsai B. Ravindran MS, et al. Nat Rev Microbiol. 2016 Jul;14(7):407-420. doi: 10.1038/nrmicro.2016.60. Epub 2016 Jun 6. Nat Rev Microbiol. 2016. PMID: 27265768 Free PMC article. Review. - How viruses use the endoplasmic reticulum for entry, replication, and assembly.
Inoue T, Tsai B. Inoue T, et al. Cold Spring Harb Perspect Biol. 2013 Jan 1;5(1):a013250. doi: 10.1101/cshperspect.a013250. Cold Spring Harb Perspect Biol. 2013. PMID: 23284050 Free PMC article. Review. - Multifaceted roles for lipids in viral infection.
Heaton NS, Randall G. Heaton NS, et al. Trends Microbiol. 2011 Jul;19(7):368-75. doi: 10.1016/j.tim.2011.03.007. Epub 2011 Apr 29. Trends Microbiol. 2011. PMID: 21530270 Free PMC article. Review. - Complex Membrane Remodeling during Virion Assembly of the 30,000-Year-Old Mollivirus Sibericum.
Quemin ER, Corroyer-Dulmont S, Baskaran A, Penard E, Gazi AD, Christo-Foroux E, Walther P, Abergel C, Krijnse-Locker J. Quemin ER, et al. J Virol. 2019 Jun 14;93(13):e00388-19. doi: 10.1128/JVI.00388-19. Print 2019 Jul 1. J Virol. 2019. PMID: 30996095 Free PMC article.
Cited by
- Multi-strain modeling of influenza vaccine effectiveness in older adults and its dependence on antigenic distance.
Urdy S, Hanke M, Toledo AI, Ratto N, Jacob E, Peyronnet E, Gourlet JB, Chaves SS, Thommes E, Coudeville L, Boissel JP, Courcelles E, Bruezière L. Urdy S, et al. Sci Rep. 2024 Nov 8;14(1):27190. doi: 10.1038/s41598-024-72716-1. Sci Rep. 2024. PMID: 39516205 Free PMC article. - Host Jump of an Exotic Fish Rhabdovirus into a New Class of Animals Poses a Disease Threat to Amphibians.
Emmenegger EJ, Bueren EK, Conway CM, Sanders GE, Hendrix AN, Schroeder T, Di Cicco E, Pham PH, Lumsden JS, Clouthier SC. Emmenegger EJ, et al. Viruses. 2024 Jul 25;16(8):1193. doi: 10.3390/v16081193. Viruses. 2024. PMID: 39205167 Free PMC article. - Editorial: Reviews in virus and host.
Ramirez-Martínez G. Ramirez-Martínez G. Front Cell Infect Microbiol. 2024 Jul 8;14:1445721. doi: 10.3389/fcimb.2024.1445721. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39040599 Free PMC article. No abstract available. - Mechanism and therapeutic potential of traditional Chinese medicine extracts in sepsis.
Fu CF, Li JL, Chen JW, Liang H, Zhao WR, He SY, Ma XW, Yang XF, Wang HL. Fu CF, et al. Front Pharmacol. 2024 Jul 3;15:1365639. doi: 10.3389/fphar.2024.1365639. eCollection 2024. Front Pharmacol. 2024. PMID: 39021837 Free PMC article. Review. - Influenza A viral burst size from thousands of infected single cells using droplet quantitative PCR (dqPCR).
Zath GK, Thomas MM, Loveday EK, Bikos DA, Sanche S, Ke R, Brooke CB, Chang CB. Zath GK, et al. PLoS Pathog. 2024 Jul 1;20(7):e1012257. doi: 10.1371/journal.ppat.1012257. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 38950082 Free PMC article.
References
- Fields, B. N., Knipe, D. M., Howley, P. M. Fields Virology 6th edn (Lippincott Williams & Wilkins, 2013).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources