Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons - PubMed (original) (raw)
Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons
Santosh Pothula et al. Neuropsychopharmacology. 2021 Mar.
Abstract
Dysregulation of the glutamatergic system and its receptors in medial prefrontal cortex (mPFC) has been implicated in major depressive disorder. Recent preclinical studies have shown that enhancing NMDA receptor (NMDAR) activity can exert rapid antidepressant-like effects. AGN-241751, an NMDAR positive allosteric modulator (PAM), is currently being tested as an antidepressant in clinical trials, but the mechanism and NMDAR subunit(s) mediating its antidepressant-like effects are unknown. We therefore used molecular, biochemical, and electrophysiological approaches to examine the cell-type-specific role of GluN2B-containing NMDAR in mediating antidepressant-like behavioral effects of AGN-241751. We demonstrate that AGN-241751 exerts antidepressant-like effects and reverses behavioral deficits induced by chronic unpredictable stress in mice. AGN-241751 treatment enhances NMDAR activity of excitatory and parvalbumin-inhibitory neurons in mPFC, activates Akt/mTOR signaling, and increases levels of synaptic proteins crucial for synaptic plasticity in the prefrontal cortex. Furthermore, cell-type-specific knockdown of GluN2B-containing NMDARs in mPFC demonstrates that GluN2B subunits on excitatory, but not inhibitory, neurons are necessary for antidepressant-like effects of AGN-241751. Together, these results demonstrate antidepressant-like actions of the NMDAR PAM AGN-241751 and identify GluN2B on excitatory neurons of mPFC as initial cellular trigger underlying these behavioral effects.
Figures
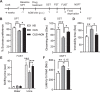
Fig. 1. AGN-241751 treatment produces dose-dependent antidepressant-like effects and activates Akt/mTOR signaling in the PFC.
A Time line of drug administration and behavioral testing. Behavioral studies were performed 24 h after vehicle or AGN-241751 (10, 50, 100, and 1000 µg/Kg; p.o.) administration to single housed mice. Dose-dependent effects of AGN-241751 on immobility time (B) in the FST (_F_4,20 = 4.20, p = 0.01), latency to feed (C) in the NSFT (_F_4,20 = 3.33, p = 0.03), and food consumption (D) in home cage feeding test (_F_4,20 = 0.50, p = 0.73). Rapid antidepressant-like effect in the FST and biochemical studies on PFC tissue samples for activation of Akt/mTOR signaling were performed 1 h after vehicle or AGN-241751 (50 µg/Kg; p.o.) treatment. AGN-241751 (50 µg/Kg; p.o.) significantly decreased immobility time in the FST (E), 1 h after drug treatment. AGN-241751 (50 µg/Kg; p.o.) significantly increased phosphorylation of Akt (G), and showed a strong tendency for increase in phosphorylation of p70s6k (F) and ERK1/2 (H), but no change in phosphorylation of eEF2 (I) in the PFC. J, K Representative western blot images for quantified proteins. Data are expressed as mean ± SEM, n = 4–6 male (B–D); 4–5 male and female (E); 8 male (F–I) mice/group, *p < 0.05, one-way ANOVA post hoc Tukey’s multiple comparison test (B–D) or Student’s (unpaired) t test (E–I).
Fig. 2. A single dose of AGN-241751 reverses CUS-induced depressive-like behaviors.
A Time line of CUS, drug administration, and behavioral testing. Behavioral studies were performed 24 h after vehicle or AGN-241751 (50 µg/Kg; p.o.) administration. Effects of stress and AGN-241751 on sucrose preference (B) in the SPT (_F_2,19 = 7.52, p = 0.004), grooming time (C) in the SST (_F_2,19 = 9.92, p = 0.001), immobility time (D) in the FST (_F_2,18 = 9.07, p = 0.002), female urine sniffing time (E) in the FUST (treatment: _F_2,36 = 5.41, p = 0.009; urine: _F_1,36 = 442, p < 0.001; interaction: _F_2,36 = 7.49, p = 0.002), and latency to feed (F) in the NSFT (_F_2,19 = 5.53, p = 0.01). Data are expressed as mean ± SEM, n = 7–8/group, *p < 0.05, **p < 0.01, ***p < 0.001, one-way (B–D, F) or two-way (E) ANOVA post hoc Tukey’s multiple comparison test.
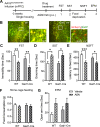
Fig. 3. AGN-241751 enhances NMDA-, but not AMPA-mediated, inward currents in excitatory and inhibitory neurons.
NMDAR-mediated inward currents were recorded from layer V pyramidal or Pvalb-inhibitory neurons of mPFC before and after bath application of either NMDA (10 µM) or AMPA (5 µM) alone or in combination with AGN-241751 (10, 30, 100, 300 nM and 1 µM). A AGN-241751 significantly increased NMDAR-mediated inward currents in layer V pyramidal neurons at 100 nM. A trend for an increase was observed at low nanomolar concentrations, but this did not reach significance. There was no effect of the highest (1 µM) concentration of AGN-241751. Data are expressed as mean ± SEM, n = 8–12 neurons from six mice (one-way ANOVA and post hoc Sidak’s multiple comparisons test, _F_5,60 = 3.06, p = 0.016). AGN-241751 (100 nM) significantly increased NMDAR-mediated inward currents in both excitatory (B) and Pvalb-expressing inhibitory neurons (D) with no effects on AMPAR-mediated inward currents. Representative traces of NMDAR- and AMPAR-mediated inward currents before and after application of AGN-241751 in excitatory neurons (C) and Pvalb-expressing inhibitory neurons (E). n = 9 (B) or 12–15 (D) neurons from five to six mice for each cell type (paired _t_-test, *p < 0.05, ***p < 0.001).
Fig. 4. Knockdown of GluN2B in the mPFC of Camk2a-Cre mice prevents antidepressant-like effects of AGN-241751.
A Schematic showing pGluN2BshRNA construct and EGFP excision after Cre-mediated recombination. B Time line of viral infusion, single housing, drug administration, and behavioral testing. C Representative images of AAV2GluN2BshRNA virus expression showing fluorescent labeling of WT neurons (yellow: colocalization of EGFP and mCherry fluorescence) and a subset of mCherry-alone expressing pyramidal neurons in the mPFC of WTCre-/AAV2B and Camk2a Cre+/AAV2B mice, respectively. AGN-241751 (50 µg/Kg; p.o.) significantly reduced immobility time (D) in the FST (treatment: _F_1,36 = 13.5, p < 0.001, genotype: _F_1,36 = 0.72, p = 0.4, interaction: _F_1,36 = 2.5, p = 0.12), increased grooming time (E) in the SST (treatment: _F_1,36 = 7.55, p = 0.009, genotype: _F_1,36 = 8.14, p = 0.007, interaction: _F_1,36 = 6.23, p = 0.02) and reduced latency to feed (F) in the NSFT (treatment: _F_1,36 = 8.03, p = 0.007, genotype: _F_1,36 = 3.67, p = 0.06, interaction: _F_1,36 = 3.21, p = 0.08) in WTCre-/AAV2B but these effects were absent in Camk2a Cre+/AAV2B mice. G There were no significant differences observed in home cage feeding across groups. H No significant effects were observed in open arm time (treatment: _F_1,35 = 1.08, p = 0.31). Data expressed as the mean ± SEM and analyzed by two-way ANOVA with Tukey’s multiple comparisons (n = 8–11 per group; *p < 0.05, **p < 0.01).
Fig. 5. Knockdown of GluN2B in the mPFC of Gad1-Cre mice does not block antidepressant-like effects of AGN-241751.
A Time line of viral infusion, single housing, drug administration, and behavioral testing. B Representative images of AAV2GluN2BshRNA virus expression showing fluorescent labeling of WT neurons (yellow: colocalization of EGFP and mCherry fluorescence) and a subset of mCherry-alone expressing inhibitory neurons in the mPFC of WTCre-/AAV2B and Gad1 Cre+/AAV2B mice, respectively. AGN-241751 (50 µg/Kg; p.o.) significantly reduced immobility time (C) in the FST (treatment: _F_1,38 = 30.4, p < 0.001, genotype: _F_1,38 = 8.91, p = 0.005, interaction: _F_1,38 = 0.3, p = 0.59), increased grooming time (D) in the SST (treatment: _F_1,35 = 17.8, p < 0.001, genotype: _F_1,35 = 0.0126, p = 0.91, interaction: _F_1,35 = 0.033, p = 0.86) and decreased latency to feed (E) in the NSFT (treatment: _F_1,38 = 28, p = 0.001, genotype: _F_1,38 = 2.77, p = 0.1, interaction: _F_1,38 = 1.18, p = 0.28) in both WTCre-/AAV2B and Gad1 Cre+/AAV2B mice. F There were no significant differences observed in home cage feeding across groups. G An increase in open arm time was observed after AGN-241751 in both WTCre-/AAV2B and Gad1 Cre+/AAV2B mice (n = 4–8 male or female mice/group for EPM; treatment: _F_1,20 = 6.49, p = 0.02). Data expressed as the Mean ± SEM and analyzed by two-way ANOVA with Tukey’s multiple comparisons (n = 8–12/group (C–F); *p < 0.05, **p < 0.01).
Similar articles
- Cell-type specific modulation of NMDA receptors triggers antidepressant actions.
Pothula S, Kato T, Liu RJ, Wu M, Gerhard D, Shinohara R, Sliby AN, Chowdhury GMI, Behar KL, Sanacora G, Banerjee P, Duman RS. Pothula S, et al. Mol Psychiatry. 2021 Sep;26(9):5097-5111. doi: 10.1038/s41380-020-0796-3. Epub 2020 Jun 2. Mol Psychiatry. 2021. PMID: 32488125 - Uncoupling DAPK1 from NMDA receptor GluN2B subunit exerts rapid antidepressant-like effects.
Li SX, Han Y, Xu LZ, Yuan K, Zhang RX, Sun CY, Xu DF, Yuan M, Deng JH, Meng SQ, Gao XJ, Wen Q, Liu LJ, Zhu WL, Xue YX, Zhao M, Shi J, Lu L. Li SX, et al. Mol Psychiatry. 2018 Mar;23(3):597-608. doi: 10.1038/mp.2017.85. Epub 2017 Apr 25. Mol Psychiatry. 2018. PMID: 28439098 Free PMC article. - NMDA receptor subunits and associated signaling molecules mediating antidepressant-related effects of NMDA-GluN2B antagonism.
Kiselycznyk C, Jury NJ, Halladay LR, Nakazawa K, Mishina M, Sprengel R, Grant SG, Svenningsson P, Holmes A. Kiselycznyk C, et al. Behav Brain Res. 2015;287:89-95. doi: 10.1016/j.bbr.2015.03.023. Epub 2015 Mar 21. Behav Brain Res. 2015. PMID: 25800971 Free PMC article. - Mechanisms of ketamine action as an antidepressant.
Zanos P, Gould TD. Zanos P, et al. Mol Psychiatry. 2018 Apr;23(4):801-811. doi: 10.1038/mp.2017.255. Epub 2018 Mar 13. Mol Psychiatry. 2018. PMID: 29532791 Free PMC article. Review. - Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition.
Miller OH, Moran JT, Hall BJ. Miller OH, et al. Neuropharmacology. 2016 Jan;100:17-26. doi: 10.1016/j.neuropharm.2015.07.028. Epub 2015 Jul 26. Neuropharmacology. 2016. PMID: 26211972 Review.
Cited by
- Research Progress on NMDA Receptor Enhancement Drugs for the Treatment of Depressive Disorder.
Liu R, Liu N, Ma L, Liu Y, Huang Z, Peng X, Zhuang C, Niu J, Yu J, Du J. Liu R, et al. CNS Drugs. 2024 Dec;38(12):985-1002. doi: 10.1007/s40263-024-01123-x. Epub 2024 Oct 8. CNS Drugs. 2024. PMID: 39379772 Review. - The Complex World of Kynurenic Acid: Reflections on Biological Issues and Therapeutic Strategy.
Stone TW, Darlington LG, Badawy AA, Williams RO. Stone TW, et al. Int J Mol Sci. 2024 Aug 20;25(16):9040. doi: 10.3390/ijms25169040. Int J Mol Sci. 2024. PMID: 39201726 Free PMC article. Review. - Glutamatergic Modulators for Major Depression from Theory to Clinical Use.
McIntyre RS, Jain R. McIntyre RS, et al. CNS Drugs. 2024 Nov;38(11):869-890. doi: 10.1007/s40263-024-01114-y. Epub 2024 Aug 16. CNS Drugs. 2024. PMID: 39150594 Free PMC article. Review. - Targeting metaplasticity mechanisms to promote sustained antidepressant actions.
Brown KA, Gould TD. Brown KA, et al. Mol Psychiatry. 2024 Apr;29(4):1114-1127. doi: 10.1038/s41380-023-02397-1. Epub 2024 Jan 4. Mol Psychiatry. 2024. PMID: 38177353 Free PMC article. Review. - Central regulation of stress-evoked peripheral immune responses.
Chan KL, Poller WC, Swirski FK, Russo SJ. Chan KL, et al. Nat Rev Neurosci. 2023 Oct;24(10):591-604. doi: 10.1038/s41583-023-00729-2. Epub 2023 Aug 25. Nat Rev Neurosci. 2023. PMID: 37626176 Free PMC article. Review.
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858. - PMC - PubMed
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53:649–59. - PubMed
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. - PubMed
- Curtin SC, Warner M, Hedegaard H. Increase in suicide in the United States, 1999–2014. NCHS Data Brief. 2016;241:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous