Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2021 Jan 1;181(1):24-31.
doi: 10.1001/jamainternmed.2020.6615.
Giovanni Dolci 3, Marco Massari 4, Domenico Franco Merlo 5, Silvio Cavuto 5, Luisa Savoldi 5, Paolo Bruzzi 6, Fabrizio Boni 7, Luca Braglia 5, Caterina Turrà 8, Pier Ferruccio Ballerini 9, Roberto Sciascia 9, Lorenzo Zammarchi 10, Ombretta Para 11, Pier Giorgio Scotton 12, Walter Omar Inojosa 12, Viviana Ravagnani 13, Nicola Duccio Salerno 14, Pier Paolo Sainaghi 15, Alessandro Brignone 16, Mauro Codeluppi 17, Elisabetta Teopompi 7, Maurizio Milesi 18, Perla Bertomoro 19, Norbiato Claudio 20, Mario Salio 21, Marco Falcone 22, Giovanni Cenderello 23, Lorenzo Donghi 24, Valerio Del Bono 25, Paolo Luigi Colombelli 26, Andrea Angheben 27, Angelina Passaro 28, Giovanni Secondo 29, Renato Pascale 30, Ilaria Piazza 31, Nicola Facciolongo 32, Massimo Costantini 33; RCT-TCZ-COVID-19 Study Group
Affiliations
- PMID: 33080005
- PMCID: PMC7577199
- DOI: 10.1001/jamainternmed.2020.6615
Randomized Controlled Trial
Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial
Carlo Salvarani et al. JAMA Intern Med. 2021.
Abstract
Importance: The coronavirus disease 2019 (COVID-19) pandemic is threatening billions of people worldwide. Tocilizumab has shown promising results in retrospective studies in patients with COVID-19 pneumonia with a good safety profile.
Objective: To evaluate the effect of early tocilizumab administration vs standard therapy in preventing clinical worsening in patients hospitalized with COVID-19 pneumonia.
Design, setting, and participants: Prospective, open-label, randomized clinical trial that randomized patients hospitalized between March 31 and June 11, 2020, with COVID-19 pneumonia to receive tocilizumab or standard of care in 24 hospitals in Italy. Cases of COVID-19 were confirmed by polymerase chain reaction method with nasopharyngeal swab. Eligibility criteria included COVID-19 pneumonia documented by radiologic imaging, partial pressure of arterial oxygen to fraction of inspired oxygen (Pao2/Fio2) ratio between 200 and 300 mm Hg, and an inflammatory phenotype defined by fever and elevated C-reactive protein.
Interventions: Patients in the experimental arm received intravenous tocilizumab within 8 hours from randomization (8 mg/kg up to a maximum of 800 mg), followed by a second dose after 12 hours. Patients in the control arm received supportive care following the protocols of each clinical center until clinical worsening and then could receive tocilizumab as a rescue therapy.
Main outcome and measures: The primary composite outcome was defined as entry into the intensive care unit with invasive mechanical ventilation, death from all causes, or clinical aggravation documented by the finding of a Pao2/Fio2 ratio less than 150 mm Hg, whichever came first.
Results: A total of 126 patients were randomized (60 to the tocilizumab group; 66 to the control group). The median (interquartile range) age was 60.0 (53.0-72.0) years, and the majority of patients were male (77 of 126, 61.1%). Three patients withdrew from the study, leaving 123 patients available for the intention-to-treat analyses. Seventeen patients of 60 (28.3%) in the tocilizumab arm and 17 of 63 (27.0%) in the standard care group showed clinical worsening within 14 days since randomization (rate ratio, 1.05; 95% CI, 0.59-1.86). Two patients in the experimental group and 1 in the control group died before 30 days from randomization, and 6 and 5 patients were intubated in the 2 groups, respectively. The trial was prematurely interrupted after an interim analysis for futility.
Conclusions and relevance: In this randomized clinical trial of hospitalized adult patients with COVID-19 pneumonia and Pao2/Fio2 ratio between 200 and 300 mm Hg who received tocilizumab, no benefit on disease progression was observed compared with standard care. Further blinded, placebo-controlled randomized clinical trials are needed to confirm the results and to evaluate possible applications of tocilizumab in different stages of the disease.
Trial registration: ClinicalTrials.gov Identifier: NCT04346355; EudraCT Identifier: 2020-001386-37.
Conflict of interest statement
Conflict of Interest Disclosures: Drs Massari, Merlo, and Costantini, Mr Cavuto, and Mss Savoldi and Turrà reported receiving nonfinancial support (provision of experimental drug and distribution to clinical sites) from Roche during the conduct of the study. Dr Falcone reported receiving speaker fees from Angelini, Merck Sharp & Dohme, Pfizer, Nordic Pharma, and Shionogi outside the submitted work. Dr Angheben reported receiving grants from Italian Ministry of Health during the conduct of the study. No other disclosures were reported.
Figures
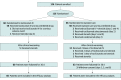
Figure 1.. Flowchart of the Study
IV indicates intravenous.
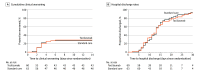
Figure 2.. Kaplan-Meier Estimates of Cumulative Clinical Worsening and Hospital Discharge
Kaplan-Meier estimates of cumulative clinical worsening (A) and hospital discharge (B).
Comment in
- Time to Reassess Tocilizumab's Role in COVID-19 Pneumonia.
Parr JB. Parr JB. JAMA Intern Med. 2021 Jan 1;181(1):12-15. doi: 10.1001/jamainternmed.2020.6557. JAMA Intern Med. 2021. PMID: 33079980 No abstract available. - Tocilizumab in Treatment for Patients With COVID-19.
Bell LCK, Pollara G. Bell LCK, et al. JAMA Intern Med. 2021 Jul 1;181(7):1019. doi: 10.1001/jamainternmed.2021.0401. JAMA Intern Med. 2021. PMID: 33818593 No abstract available. - Tocilizumab in Treatment for Patients With COVID-19.
Rossi JF, Chiang HC, Klein B. Rossi JF, et al. JAMA Intern Med. 2021 Jul 1;181(7):1018-1019. doi: 10.1001/jamainternmed.2021.0395. JAMA Intern Med. 2021. PMID: 33818612 No abstract available. - Tocilizumab in Treatment for Patients With COVID-19.
Yang C, Liu M. Yang C, et al. JAMA Intern Med. 2021 Jul 1;181(7):1017-1018. doi: 10.1001/jamainternmed.2021.0392. JAMA Intern Med. 2021. PMID: 33818614 No abstract available.
Similar articles
- Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.
Emadi A, Chua JV, Talwani R, Bentzen SM, Baddley J. Emadi A, et al. Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article. - Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial.
Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P; CORIMUNO-19 Collaborative Group. Hermine O, et al. JAMA Intern Med. 2021 Jan 1;181(1):32-40. doi: 10.1001/jamainternmed.2020.6820. JAMA Intern Med. 2021. PMID: 33080017 Free PMC article. Clinical Trial. - Testing the efficacy and safety of BIO101, for the prevention of respiratory deterioration, in patients with COVID-19 pneumonia (COVA study): a structured summary of a study protocol for a randomised controlled trial.
Dioh W, Chabane M, Tourette C, Azbekyan A, Morelot-Panzini C, Hajjar LA, Lins M, Nair GB, Whitehouse T, Mariani J, Latil M, Camelo S, Lafont R, Dilda PJ, Veillet S, Agus S. Dioh W, et al. Trials. 2021 Jan 11;22(1):42. doi: 10.1186/s13063-020-04998-5. Trials. 2021. PMID: 33430924 Free PMC article. - Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis.
Tleyjeh IM, Kashour Z, Damlaj M, Riaz M, Tlayjeh H, Altannir M, Altannir Y, Al-Tannir M, Tleyjeh R, Hassett L, Kashour T. Tleyjeh IM, et al. Clin Microbiol Infect. 2021 Feb;27(2):215-227. doi: 10.1016/j.cmi.2020.10.036. Epub 2020 Nov 5. Clin Microbiol Infect. 2021. PMID: 33161150 Free PMC article. - Tocilizumab in patients hospitalized with COVID-19 pneumonia: systematic review and meta-analysis of randomized controlled trials.
Gupta S, Padappayil RP, Bansal A, Daouk S, Brown B. Gupta S, et al. J Investig Med. 2022 Jan;70(1):55-60. doi: 10.1136/jim-2021-002001. Epub 2021 Sep 24. J Investig Med. 2022. PMID: 34561232 Free PMC article.
Cited by
- COVID-19 and pulmonary fibrosis: A potential role for lung epithelial cells and fibroblasts.
John AE, Joseph C, Jenkins G, Tatler AL. John AE, et al. Immunol Rev. 2021 Jul;302(1):228-240. doi: 10.1111/imr.12977. Epub 2021 May 24. Immunol Rev. 2021. PMID: 34028807 Free PMC article. Review. - Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study.
Cavalli G, Larcher A, Tomelleri A, Campochiaro C, Della-Torre E, De Luca G, Farina N, Boffini N, Ruggeri A, Poli A, Scarpellini P, Rovere-Querini P, Tresoldi M, Salonia A, Montorsi F, Landoni G, Castagna A, Ciceri F, Zangrillo A, Dagna L. Cavalli G, et al. Lancet Rheumatol. 2021 Apr;3(4):e253-e261. doi: 10.1016/S2665-9913(21)00012-6. Epub 2021 Feb 3. Lancet Rheumatol. 2021. PMID: 33655218 Free PMC article. - COVID-19 subphenotypes at hospital admission are associated with mortality: a cross-sectional study.
Dubowski K, Braganza GT, Bozack A, Colicino E, DeFelice N, McGuinn L, Maru D, Lee AG. Dubowski K, et al. Ann Med. 2023 Dec;55(1):12-23. doi: 10.1080/07853890.2022.2148733. Ann Med. 2023. PMID: 36444856 Free PMC article. - Plasma from patients with bacterial sepsis or severe COVID-19 induces suppressive myeloid cell production from hematopoietic progenitors in vitro.
Reyes M, Filbin MR, Bhattacharyya RP, Sonny A, Mehta A, Billman K, Kays KR, Pinilla-Vera M, Benson ME, Cosimi LA, Hung DT, Levy BD, Villani AC, Sade-Feldman M, Baron RM, Goldberg MB, Blainey PC, Hacohen N. Reyes M, et al. Sci Transl Med. 2021 Jun 16;13(598):eabe9599. doi: 10.1126/scitranslmed.abe9599. Epub 2021 Jun 8. Sci Transl Med. 2021. PMID: 34103408 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials