Low-Dose Berberine Attenuates the Anti-Breast Cancer Activity of Chemotherapeutic Agents via Induction of Autophagy and Antioxidation - PubMed (original) (raw)
Low-Dose Berberine Attenuates the Anti-Breast Cancer Activity of Chemotherapeutic Agents via Induction of Autophagy and Antioxidation
Bing Han et al. Dose Response. 2020.
Abstract
Berberine (BBR), a major active component of Rhizoma coptidis, is one of the most promising agents for breast cancer adjuvant therapy. It is well accepted that BBR could exhibit remarkable anticancer efficacy with few side effects, and when treated with chemotherapeutic agents in combination, BBR could enhance the chemosensitivity of cancer cells. Our previous study reported that low-dose BBR (LDB) induced hormetic effect and attenuated the anticancer activity of chemotherapeutic agents. However, the underlying mechanisms are still unclear. In this study, we confirmed that LDB could promote cancer cell proliferation and antagonize the anti-breast cancer activities of chemotherapeutic agents. And the mechanisms were proved to be induction of autophagy and antioxidation by LDB. Our results showed that LDB could mildly induce reactive oxygen species, raise the level of autophagy by promoting the phosphorylation of adenosine monophosphate-activated protein kinase, and promote antioxidant enzymes expression through activating nuclear factor erythroid 2-related factor 2 in breast cancer cells. These findings revealed a potential negative impact of BBR on its adjuvant anti-breast cancer therapy, providing guidance for a safe and effective use of naturally originated medicines in the clinic.
Keywords: ROS; autophagy; berberine; breast cancer; chemotherapy; hormesis.
© The Author(s) 2020.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Figure 1.
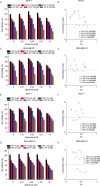
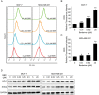
Effect of BBR on chemotherapeutic agents induced cytotoxicity in breast cancer cells. MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR and 5-FU (A-D) or CPT (E-G), before the determination of cell viability by MTT assay (A, C, E, G) and the combination index analysis by CompuSyn software 34 (B, D, F, H). Data are expressed as mean ± SD (n = 3). CI < 1 indicates synergy, CI = 1 indicates an additive effect, and CI > 1 indicates antagonism. BBR indicates berberine; 5-FU, 5-fluorouracil; CPT, camptothecin; MTT, thiazolyl blue tetrazolium bromide; CI, combination index.
Figure 2.
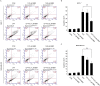
Effect of LDB on CPT induced apoptosis in breast cancer cells. MCF-7 (A, B) and MDA-MB-231 (C, D) cells were treated with CPT or LDB, either alone or in combination, before apoptosis evaluation by Annexin V-FITC/PI double staining. Data are expressed as mean ± SD (n = 3). *P < .05, **P < .01, compared with the CPT group. CPT indicates camptothecin; LDB, low dose BBR; PI, propidium iodide.
Figure 3.
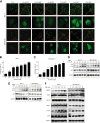
BBR induced autophagy in breast cancer cells. A, The EGFP-LC3B stably transfected MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR, before quantitative analysis performed by INCell analyzer 2000. B, C, The autophagic cells (%) = total number of EGFP-LC3 positive cells/total number of viable cells × 100%. * P< .05, ** P < .01, compared with the CON (0 μM) group. D, MCF-7 and MDA-MB-231 cells were treated with LDB (0.31 μM) for 0, 4, 8, 12, and 24 hours. E, F, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR (0.31-40 μM) for 24 hours. Total protein of cell lysates was extracted and subjected to Western blot analysis. GAPDH was used as the internal control. Data are expressed as mean ± SD (n = 3). BBR indicates berberine; EGFP-LC3B, enhanced GFP-microtubule-associated protein 1A/1B light chain 3 beta; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LDB, low-dose BBR, AMPK, adenosine monophosphate-activated protein kinase; ULK1, unc-51 like autophagy activating kinase 1.
Figure 4.
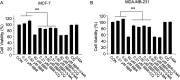
LDB promoted the proliferation of human breast cancer cells through autophagy induction. MCF-7 and MDA-MB-231cells were treated with different concentrations of BBR with or without autophagy inhibitors (CQ or 3-MA), before the determination of cell viability by MTT assay. Data are expressed as mean ± SD (n = 3). * P < .05, **P < .01, compared with the autophagy inhibitors free groups. BBR indicates berberine; LDB, low-dose BBR; CQ, chloroquine; 3-MA, 3-methyladenine, MTT, thiazolyl blue tetrazolium bromide.
Figure 5.
BBR stimulated ROS generation and upregulated antioxidant proteins. A, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR, before ROS production evaluation with DCFH-DA dye. B and C, The quantitative analysis of ROS relative fluorescence intensity. Data are expressed as mean ± SD (n = 3). * P < .05, **P < .01, compared with the CON (0 μM) group. D, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR. Total protein of cell lysates was extracted and subjected to Western blot analysis. GAPDH was used as the internal control. BBR indicates berberine; ROS, reactive oxygen species; HO-1, heme oxygenase-1; SOD2, superoxide dismutase 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Figure 6.
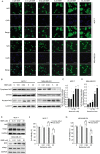
LDB promoted the proliferation of human breast cancer cells through Nrf2 activation. A, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR, before immunofluorescence and localization analysis by INCell analyzer 2000 system. Top panel: Green fluorescence showing Nrf2 localization; middle panel: stained nucleus with DAPI. B, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR. Desired proteins were extracted from the nucleus and cytoplasm, respectively, and subjected to Western blot analysis. GAPDH was used as the internal control for cytoplasm-Nrf2, and Lamin B was used as the internal control for nucleus-Nrf2. C and D, The quantitative analysis of Nrf2 relative intensities in (B). The density of each band was quantified by Image J, and the relative density ratio of each protein was calculated accordingly. Data are expressed as mean ± SD (n = 3). *P < .05, ** P < .01, compared with the CON (0 μM) group. E, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR for 24 hours. Total protein of cell lysates was extracted and subjected to Western blot analysis. GAPDH was used as the internal control. F, MCF-7 and MDA-MB-231 cells were transfected with siRNA against Nrf2 and negative control siRNA. The cells were treated with different concentrations of BBR, before the determination of cell viability by MTT assay. Data are expressed as mean ± SD (n = 3). * P < .05, ** P < .01, compared with the Control siRNA groups. BBR indicates berberine; LDB, low-dose BBR; Nrf2, nuclear factor erythroid 2-related factor 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MTT, thiazolyl blue tetrazolium bromide; siRNA, small interfering RNA.
Figure 7.
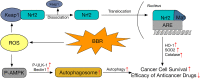
Schematic diagram showing the proposed mechanisms for the enhancement of cancer cell survival and antagonism of anticancer drugs by low-dose berberine.
Similar articles
- Hormetic Effect of Berberine Attenuates the Anticancer Activity of Chemotherapeutic Agents.
Bao J, Huang B, Zou L, Chen S, Zhang C, Zhang Y, Chen M, Wan JB, Su H, Wang Y, He C. Bao J, et al. PLoS One. 2015 Sep 30;10(9):e0139298. doi: 10.1371/journal.pone.0139298. eCollection 2015. PLoS One. 2015. PMID: 26421434 Free PMC article. - Berberine, a natural antidiabetes drug, attenuates glucose neurotoxicity and promotes Nrf2-related neurite outgrowth.
Hsu YY, Tseng YT, Lo YC. Hsu YY, et al. Toxicol Appl Pharmacol. 2013 Nov 1;272(3):787-96. doi: 10.1016/j.taap.2013.08.008. Epub 2013 Aug 15. Toxicol Appl Pharmacol. 2013. PMID: 23954465 - Comparison of anti-inflammatory effects of berberine, and its natural oxidative and reduced derivatives from Rhizoma Coptidis in vitro and in vivo.
Li CL, Tan LH, Wang YF, Luo CD, Chen HB, Lu Q, Li YC, Yang XB, Chen JN, Liu YH, Xie JH, Su ZR. Li CL, et al. Phytomedicine. 2019 Jan;52:272-283. doi: 10.1016/j.phymed.2018.09.228. Epub 2018 Oct 1. Phytomedicine. 2019. PMID: 30599908 - The Anti-Cancer Mechanisms of Berberine: A Review.
Wang Y, Liu Y, Du X, Ma H, Yao J. Wang Y, et al. Cancer Manag Res. 2020 Jan 30;12:695-702. doi: 10.2147/CMAR.S242329. eCollection 2020. Cancer Manag Res. 2020. PMID: 32099466 Free PMC article. Review. - HGSD attenuates neuronal apoptosis through enhancing neuronal autophagy in the brain of diabetic mice: The role of AMP-activated protein kinase.
Xue H, Ji Y, Wei S, Yu Y, Yan X, Liu S, Zhang M, Yao F, Lan X, Chen L. Xue H, et al. Life Sci. 2016 May 15;153:23-34. doi: 10.1016/j.lfs.2016.04.004. Epub 2016 Apr 8. Life Sci. 2016. PMID: 27067476 Review.
Cited by
- Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications.
Tian E, Sharma G, Dai C. Tian E, et al. Antioxidants (Basel). 2023 Oct 19;12(10):1883. doi: 10.3390/antiox12101883. Antioxidants (Basel). 2023. PMID: 37891961 Free PMC article. Review. - Berberine as a Potential Anticancer Agent: A Comprehensive Review.
Rauf A, Abu-Izneid T, Khalil AA, Imran M, Shah ZA, Emran TB, Mitra S, Khan Z, Alhumaydhi FA, Aljohani ASM, Khan I, Rahman MM, Jeandet P, Gondal TA. Rauf A, et al. Molecules. 2021 Dec 4;26(23):7368. doi: 10.3390/molecules26237368. Molecules. 2021. PMID: 34885950 Free PMC article. Review. - A specific super-enhancer actuated by berberine regulates EGFR-mediated RAS-RAF1-MEK1/2-ERK1/2 pathway to induce nasopharyngeal carcinoma autophagy.
Wu Y, Jia Q, Tang Q, Chen L, Deng H, He Y, Tang F. Wu Y, et al. Cell Mol Biol Lett. 2024 Jun 28;29(1):92. doi: 10.1186/s11658-024-00607-4. Cell Mol Biol Lett. 2024. PMID: 38943090 Free PMC article. - The Protective Effect of a Human Umbilical Cord Mesenchymal Stem Cell Supernatant on UVB-Induced Skin Photodamage.
Cheng L, Liu J, Wang Q, Hu H, Zhou L. Cheng L, et al. Cells. 2024 Jan 15;13(2):156. doi: 10.3390/cells13020156. Cells. 2024. PMID: 38247847 Free PMC article. - Protective effects of naringin on glucocorticoid-induced osteoporosis through regulating the PI3K/Akt/mTOR signaling pathway.
Ge X, Zhou G. Ge X, et al. Am J Transl Res. 2021 Jun 15;13(6):6330-6341. eCollection 2021. Am J Transl Res. 2021. PMID: 34306372 Free PMC article.
References
- Bai XP, Ni J, Beretov J, Graham P, Li Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev. 2018;69:152–163. doi:10.1016/j.ctrv.2018.07.004 - PubMed
- Ou HT, Chung WP, Su PF, et al. Health-related quality of life associated with different cancer treatments in Chinese breast cancer survivors in Taiwan. Eur J Cancer Care. 2019;28(4):e13069 doi:10.1111/ecc.13069 - PubMed
- Imenshahidi M, Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): a clinical review. Phytother Res. 2019;33(3):504–523. doi:10.1002/ptr.6252 - PubMed
LinkOut - more resources
Full Text Sources