Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes - PubMed (original) (raw)
[Preprint]. 2020 Nov 13:2020.10.29.361048.
doi: 10.1101/2020.10.29.361048.
Bhesh Raj Sharma 1, Shraddha Tuladhar 1, Evan Peter Williams 2, Lillian Zalduondo 2, Parimal Samir 1, Min Zheng 1, Balamurugan Sundaram 1, Balaji Banoth 1, R K Subbarao Malireddi 1, Patrick Schreiner 3, Geoffrey Neale 4, Peter Vogel 5, Richard Webby 6, Colleen Beth Jonsson 2, Thirumala-Devi Kanneganti 1
Affiliations
- PMID: 33140051
- PMCID: PMC7605562
- DOI: 10.1101/2020.10.29.361048
Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes
Rajendra Karki et al. bioRxiv. 2020.
Update in
- Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes.
Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, Schreiner P, Neale G, Vogel P, Webby R, Jonsson CB, Kanneganti TD. Karki R, et al. Cell. 2021 Jan 7;184(1):149-168.e17. doi: 10.1016/j.cell.2020.11.025. Epub 2020 Nov 19. Cell. 2021. PMID: 33278357 Free PMC article.
Abstract
The COVID-19 pandemic has caused significant morbidity and mortality. Currently, there is a critical shortage of proven treatment options and an urgent need to understand the pathogenesis of multi-organ failure and lung damage. Cytokine storm is associated with severe inflammation and organ damage during COVID-19. However, a detailed molecular pathway defining this cytokine storm is lacking, and gaining mechanistic understanding of how SARS-CoV-2 elicits a hyperactive inflammatory response is critical to develop effective therapeutics. Of the multiple inflammatory cytokines produced by innate immune cells during SARS-CoV-2 infection, we found that the combined production of TNF-α and IFN-γ specifically induced inflammatory cell death, PANoptosis, characterized by gasdermin-mediated pyroptosis, caspase-8-mediated apoptosis, and MLKL-mediated necroptosis. Deletion of pyroptosis, apoptosis, or necroptosis mediators individually was not sufficient to protect against cell death. However, cells deficient in both RIPK3 and caspase-8 or RIPK3 and FADD were resistant to this cell death. Mechanistically, the JAK/STAT1/IRF1 axis activated by TNF-α and IFN-γ co-treatment induced iNOS for the production of nitric oxide. Pharmacological and genetic deletion of this pathway inhibited pyroptosis, apoptosis, and necroptosis in macrophages. Moreover, inhibition of PANoptosis protected mice from TNF-α and IFN-γ-induced lethal cytokine shock that mirrors the pathological symptoms of COVID-19. In vivo neutralization of both TNF-α and IFN-γ in multiple disease models associated with cytokine storm showed that this treatment provided substantial protection against not only SARS-CoV-2 infection, but also sepsis, hemophagocytic lymphohistiocytosis, and cytokine shock models, demonstrating the broad physiological relevance of this mechanism. Collectively, our findings suggest that blocking the cytokine-mediated inflammatory cell death signaling pathway identified here may benefit patients with COVID-19 or other cytokine storm-driven syndromes by limiting inflammation and tissue damage. The findings also provide a molecular and mechanistic description for the term cytokine storm. Additionally, these results open new avenues for the treatment of other infectious and autoinflammatory diseases and cancers where TNF-α and IFN-γ synergism play key pathological roles.
Keywords: COVID-19; FADD; IFN-γ; IRF1; MLKL; PANoptosis; RIPK1; RIPK3; SARS-CoV-2; STAT1; TNF-α; acute respiratory distress syndrome (ARDS); apoptosis; caspase; caspase-8; cell death; cytokine shock syndromes; cytokine storm; gasdermin D; gasdermin E; hemophagocytic lymphohistiocytosis (HLH); iNOS; inflammatory cytokines; macrophage activation syndrome (MAS); necroptosis; nitric oxide; pyroptosis; sepsis; systemic inflammatory response syndrome (SIRS).
Conflict of interest statement
DECLARATION OF INTERESTS St. Jude Children’s Research hospital filed a provisional patent application on TNF-α and IFN-γ signaling described in this study, listing R.K. and T.-D.K. as inventors (Serial No. 63/106,012).
Figures
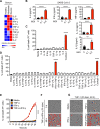
Figure 1.. Pro-inflammatory cytokines are increased in COVID-19, and co-treatment of TNF-α and IFN-γ induces cell death
(A) Heatmap depicting the levels of pro-inflammatory cytokines in serum of patients with COVID-19 and healthy people (Lucas et al., 2020). (B) Pro-inflammatory cytokines released from PBMCs infected with SARS-CoV-2. (C) Percent of BMDMs that are dead 48 h after cytokine treatment using the IncuCyte imaging system and propidium iodide (PI) staining. “Cocktail-1” contained all 8 cytokines (IL-6, IL-18, IFN-γ, IL-15, TNF-α, IL-1α, IL-1β, and IL-2). (D) Percent of BMDMs that are dead 48 h after treatment with the indicated combination of cytokines. (E) Real-time analysis of cell death in BMDMs treated with the indicated cytokines. (F, G) Representative images of cell death in BMDMs (F) and THP-1 cells (G) after 48 h of the indicated treatments. Scale bar, 50 μm. Data are representative of at least three independent experiments. **P < 0.01; ****P < 0.0001. Analysis was performed using the one-way ANOVA (B–D) or the two-way ANOVA (E). Significance asterisks in C and D indicate the comparison to the media-treated control. Data are shown as mean ± SEM (B–E). See also Figure S1.
Figure 2.. Cytokine shock by TNF-α and IFN-γ mirrors COVID-19 symptoms
(A) Survival of 6- to 8-week-old WT mice after i.p. injection of PBS (n = 10), IFN-γ (n = 12), TNF-α (n = 15), or TNF-α+IFN-γ (n = 15). (B) H/E staining, TUNEL, and cleaved caspase-3 (Clvd CASP3) immuno-staining of colon samples from PBS or TNF-α+IFN-γ injected mice for 5 h. Red arrows indicate stained cells. (C–E) Analysis of (C) serum levels of LDH, ALT, AST, blood urea nitrogen (BUN), and ferritin; (D) the number of thrombocytes, plateletcrit (PCT), RBC count, hematocrit (HCT), and hemoglobin (Hb) concentration in the blood; and (E) the percentage of macrophages, neutrophils, T cells, and B cells and the neutrophil-to-lymphocyte ratio (NLR) in the blood of PBS or TNF-α and IFN-γ injected mice for 5 h. Data are representative of at least three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Analysis was performed using the survival curve comparison (log-Rank [Mantel-Cox] test) (A) or the t test (C–E). Data are shown as mean ± SEM (C–E). See also Figure S2.
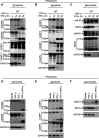
Figure 3.. Co-treatment of TNF-α and IFN-γ induces PANoptosis
(A–C) Immunoblot analysis of (A) pro- (P53), activated (P30), and inactivated (P20) GSDMD, pro- (P53) and activated (P34) GSDME, pro- (P45) and activated (P20) CASP1, and pro- (P43) and cleaved CASP-11 (P36 and P26); (B) pro- (P35) and cleaved CASP3 (P19 and P17), pro- (P35) and cleaved CASP-7 (P20), pro- (P55) and cleaved CASP8 (P18), and pro- (P49) and cleaved CASP9 (P18); and (C) phosphorylated MLKL (pMLKL), total MLKL (tMLKL), phosphorylated RIPK1 (pRIPK1), and pro- (P75) and cleaved (P30) RIPK1 in BMDMs co-treated with TNF-α and IFN-γ. (D–F) Immunoblot analysis of (D) GSDMD and GSDME; (E) CASP3, CASP7, and CASP8; and (F) pMLKL and tMLKL in BMDMs after treatment with TNF-α alone, IFN-γ alone, or co-treatment with TNF-α and IFN-γ for 36 h. GAPDH was used as the internal control. Asterisks denote a nonspecific band. Data are representative of at least three independent experiments.
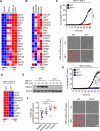
Figure 4.. IRF1 and NOS2 mediate TNF-α and IFN-γ-induced inflammatory cell death
(A) Heatmap depicting the expression levels of type II IFN-responsive genes in WT BMDMs treated with IFN-γ alone or co-treated with TNF-α and IFN-γ for 16 h relative to their expression in untreated (Mock) BMDMs. (B) Heatmap depicting the expression levels of type II IFN-responsive genes in patients with moderate, severe, and critical COVID-19 relative to their expression in healthy patients (Hadjadj et al., 2020). (C) Real-time analysis of cell death in TNF-α and IFN-γ co-treated WT and _Irf1_−/− BMDMs. Representative images of cell death are shown at 0 h and after 48 h of TNF-α and IFN-γ treatment. (D) Heatmap depicting the expression levels of the most downregulated genes in _Irf1_−/− BMDMs co-treated with TNF-α and IFN-γ for 16 h relative to their expression in WT treated BMDMs. (E) Immunoblot analysis of iNOS in WT and _Irf1_−/− BMDMs co-treated with TNF-α and IFN-γ. Actin was used as the internal control. (F) Expression analysis of NOS2 in patients with moderate, severe, and critical COVID-19 relative to the expression in healthy patients (Hadjadj et al., 2020). (G) Real-time analysis of cell death in WT, _Irf1_−/−, and _Nos2_−/− BMDMs during co-treatment with TNF-α and IFN-γ. Representative images of cell death are shown at 0 h and after 48 h of TNF-α and IFN-γ treatment. Scale bar, 50 μm. Data are representative of at least three independent experiments. **P < 0.01; ***P < 0.001; ****P < 0.0001. Analysis was performed using the one-way ANOVA (F) or two-way ANOVA (C and G). Data are shown as mean ± SEM (C, F, and G). See also Figure S3–S5.
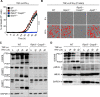
Figure 5.. Caspase-8 drives PANoptosis induced by co-treatment with TNF-α and IFN-γ
(A) Real-time analysis of cell death in WT, _Ripk3_−/−, _Ripk3_−/−_Casp8_−/−, and _Apaf1_−/− BMDMs co-treated with TNF-α and IFN-γ. (B) Representative images of cell death in WT, _Ripk3_−/−, _Ripk3_−/− _Casp8_−/−, and _Apaf1_−/− BMDMs. Scale bar, 50 μm. (C, D) Immunoblot analysis of (C) pro- (P55) and cleaved CASP8 (P18), pro- (P35) and cleaved CASP3 (P19 and P17), and pro- (P35) and cleaved CASP-7 (P20); and (D) pro- (P53) and activated (P34) GSDME, phosphorylated MLKL (pMLKL), and total MLKL (tMLKL) in WT and _Ripk3_−/−_Casp8_−/− BMDMs co-treated with TNF-α and IFN-γ. GAPDH was used as the internal control. Asterisks denote a nonspecific band. Data are representative of at least three independent experiments. ****P < 0.0001. Analysis was performed using the two-way ANOVA. Data are shown as mean ± SEM (A). See also Figures S6 and S7.
Figure 6.. Inhibition of PANoptosis provides protection against TNF-α and IFN-γ-driven lethality in mice
(A, B) Survival of 6- to 8-week-old (A) WT (n = 10) and _Stat1_−/− (n = 10) mice; and (B) WT (n = 10), _Ripk3_−/− (n = 12), and _Ripk3_−/−_Casp8_−/− (n = 15) mice after i.p. injection of TNF-α and IFN-γ. (C–E) Analysis of (C) serum levels of LDH, ALT, and AST; (D) percentage of T cells in blood; (E) the number of thrombocytes and plateletcrit (PCT) in the blood; and (F) RBC count, hematocrit (HCT), and hemoglobin (Hb) concentration in the blood of WT, _Stat1_−/−, and _Ripk3_−/−_Casp8_−/− mice injected intraperitoneally with PBS or TNF-α and IFN-γ at 5 h post-treatment. Data are representative of two independent experiments. Data are shown as mean ± SEM (C–F). *P < 0.05; **P < 0.01; ****P < 0.0001. Analysis was performed using the survival curve comparison (log-Rank [Mantel-Cox] test) (A and B) or the two-way ANOVA (C–F).
Figure 7.. Blocking TNF-α and IFN-γ provides protection in disease models associated with cytokine storm: SARS-CoV-2 infection, cytokine shock, sepsis, and HLH
(A) Survival of 6- to 8-week-old WT mice injected with isotype control (n = 10) or neutralizing antibodies against TNF-α and IFN-γ (n = 14) 12 h before i.p. injection of TNF-α and IFN-γ. (B) Survival of 7- to 8-week-old K18-hACE2 transgenic mice injected with isotype control (n = 12) or neutralizing antibodies against TNF-α and IFN-γ (n = 13) on day 1, 3, and 4 after infection with SARS-CoV-2 (2 × 104 pfu/mouse). (C) Survival of 7- to 8-week-old WT mice injected intraperitoneally with poly I:C (10 mg/kg body weight) followed 24 h later by i.p. injection of PBS (n = 15), isotype control (n = 15), neutralizing antibody against TNF-α (n = 15), neutralizing antibody against IFN-γ (n = 15), or neutralizing antibodies against both TNF-α and IFN-γ (n = 15). Mice were then challenged with LPS (5 mg/kg body weight) 1 h after the treatments. (D) Survival of 7- to 8-week-old WT mice injected with PBS (n = 17), isotype control (n = 10), neutralizing antibody against TNF-α (n = 17), neutralizing antibody against IFN-γ (n = 17), or neutralizing antibodies against both TNF-α and IFN-γ (n = 18) 30 min and 6 h after intraperitoneal injection of a lethal dose of LPS (20 mg/kg body weight). (E) Schematic overview of the mechanism of TNF-α and IFN-γ-induced pathology and the inflammatory cell death pathway with strategies for potential therapeutics. Data are pooled from two independent experiments (A–D). *P < 0.05; ***P < 0.001; ****P < 0.0001. Analysis was performed using the survival curve comparison (log-Rank [Mantel-Cox] test).
Similar articles
- Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes.
Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, Schreiner P, Neale G, Vogel P, Webby R, Jonsson CB, Kanneganti TD. Karki R, et al. Cell. 2021 Jan 7;184(1):149-168.e17. doi: 10.1016/j.cell.2020.11.025. Epub 2020 Nov 19. Cell. 2021. PMID: 33278357 Free PMC article. - Synergism of TNF-α and IFN-β triggers human airway epithelial cells death by apoptosis and pyroptosis.
Sun R, Jiang K, Zeng C, Zhu R, Chu H, Liu H, Du J. Sun R, et al. Mol Immunol. 2023 Jan;153:160-169. doi: 10.1016/j.molimm.2022.12.002. Epub 2022 Dec 9. Mol Immunol. 2023. PMID: 36508750 Free PMC article. - Helminth alleviates COVID-19-related cytokine storm in an IL-9-dependent way.
Cao Z, Wang J, Liu X, Liu Y, Li F, Liu M, Chiu S, Jin X. Cao Z, et al. mBio. 2024 Jun 12;15(6):e0090524. doi: 10.1128/mbio.00905-24. Epub 2024 May 10. mBio. 2024. PMID: 38727220 Free PMC article. - Innate immunity, cytokine storm, and inflammatory cell death in COVID-19.
Karki R, Kanneganti TD. Karki R, et al. J Transl Med. 2022 Nov 22;20(1):542. doi: 10.1186/s12967-022-03767-z. J Transl Med. 2022. PMID: 36419185 Free PMC article. Review.
Cited by
- Targeting Epigenetic Mechanisms in Vascular Aging.
Lin Z, Ding Q, Li X, Feng Y, He H, Huang C, Zhu Y. Lin Z, et al. Front Cardiovasc Med. 2022 Jan 4;8:806988. doi: 10.3389/fcvm.2021.806988. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35059451 Free PMC article. Review. - The endogenous cellular protease inhibitor SPINT2 controls SARS-CoV-2 viral infection and is associated to disease severity.
Ramirez Alvarez C, Kee C, Sharma AK, Thomas L, Schmidt FI, Stanifer ML, Boulant S, Herrmann C. Ramirez Alvarez C, et al. PLoS Pathog. 2021 Jun 28;17(6):e1009687. doi: 10.1371/journal.ppat.1009687. eCollection 2021 Jun. PLoS Pathog. 2021. PMID: 34181691 Free PMC article.
References
- Albina J.E., and Reichner J.S. (1998). Role of nitric oxide in mediation of macrophage cytotoxicity and apoptosis. Cancer Metastasis Rev 17, 39–53. - PubMed
- Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N., and Justet A. (2020). Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis 79, 1381–1382. - PubMed
- Bailey A., Pope T.W., Moore S.A., and Campbell C.L. (2007). The tragedy of TRIUMPH for nitric oxide synthesis inhibition in cardiogenic shock: where do we go from here? Am J Cardiovasc Drugs 7, 337–345. - PubMed
- Belkhelfa M., Rafa H., Medjeber O., Arroul-Lammali A., Behairi N., Abada-Bendib M., Makrelouf M., Belarbi S., Masmoudi A.N., Tazir M., et al. (2014). IFN-gamma and TNF-alpha are involved during Alzheimer disease progression and correlate with nitric oxide production: a study in Algerian patients. J Interferon Cytokine Res 34, 839–847. - PubMed
Publication types
Grants and funding
- R35 CA253095/CA/NCI NIH HHS/United States
- R01 AI101935/AI/NIAID NIH HHS/United States
- R01 AI124346/AI/NIAID NIH HHS/United States
- R37 AI101935/AI/NIAID NIH HHS/United States
- R01 AR056296/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous