Redox-informed models of global biogeochemical cycles - PubMed (original) (raw)
Review
Redox-informed models of global biogeochemical cycles
Emily J Zakem et al. Nat Commun. 2020.
Abstract
Microbial activity mediates the fluxes of greenhouse gases. However, in the global models of the marine and terrestrial biospheres used for climate change projections, typically only photosynthetic microbial activity is resolved mechanistically. To move forward, we argue that global biogeochemical models need a theoretically grounded framework with which to constrain parameterizations of diverse microbial metabolisms. Here, we explain how the key redox chemistry underlying metabolisms provides a path towards this goal. Using this first-principles approach, the presence or absence of metabolic functional types emerges dynamically from ecological interactions, expanding model applicability to unobserved environments."Nothing is less real than realism. It is only by selection, by elimination, by emphasis, that we get at the real meaning of things." -Georgia O'Keefe.
Conflict of interest statement
The authors declare no competing interests.
Figures
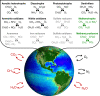
Fig. 1. Key microbially driven redox transformations that mediate the atmospheric fluxes of climatically relevant gases.
Radiatively active gases are notated with red type. The processes in black type are represented in some way (though not necessarily with electron balancing) in both the marine and terrestrial biospheres in earth system models within the Coupled Model Intercomparison Project (land: NCAR Community Earth System Model; ocean: GFDL COBALTv2), which are used for projections of climate change in reports by the Intergovernmental Panel on Climate Change. Processes in green type are represented in only the terrestrial model. Current models do not yet include other relevant reactions, some of which are represented in gray type, such as anaerobic ammonia oxidation (anammox), the marine production and consumption of methane, the redox cycling of iron, manganese, and other metals, and the methane-relevant redox chemistry of phosphorus. COBALTv2 does account for sulfate reduction in marine sediments, but sulfate is not represented. Image courtesy of NASA.
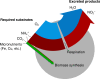
Fig. 2. Schematic of a single cell represented as a metabolic functional type carrying out the aerobic oxidation of ammonia.
The redox balance informs the elemental ratios of substrates utilized, biomass synthesized, and waste products excreted (Table 1).
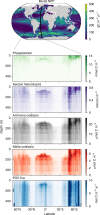
Fig. 3. Solutions from a global simulation resolving multiple metabolic functional types.
Net primary productivity (NPP), the biomasses of the metabolic functional types, and the sinking particulate organic carbon (POC) flux are resolved along a transect of a global microbial ecosystem model coupled with an estimate of the ocean circulation (Darwin-MITgcm).
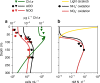
Fig. 4. Model simulation and observations of the marine nitrification system.
Biogeochemistry is driven by microbial metabolic functional types in a vertical water column model. Lines are model solutions, and marked points are observations from two stations in the Pacific Ocean, (see Supplementary Fig. 2 for more detail) (a). Chlorophyll a concentrations and abundances of ammonia-oxidizing organisms (AOO) and nitrite-oxidizing organisms (NOO). Observed abundances are of the 16S rRNA abundances of archaeal Marine Group I and _Nitrospina_-like bacteria,. Model abundances are converted from biomass with 0.1 fmol N cell-1 for AOO, 0.2 fmol N cell-1 for NOO, and one gene copy per cell. (b). Light (solar irradiance) and bulk nitrification rates.
Similar articles
- The terrestrial biosphere as a net source of greenhouse gases to the atmosphere.
Tian H, Lu C, Ciais P, Michalak AM, Canadell JG, Saikawa E, Huntzinger DN, Gurney KR, Sitch S, Zhang B, Yang J, Bousquet P, Bruhwiler L, Chen G, Dlugokencky E, Friedlingstein P, Melillo J, Pan S, Poulter B, Prinn R, Saunois M, Schwalm CR, Wofsy SC. Tian H, et al. Nature. 2016 Mar 10;531(7593):225-8. doi: 10.1038/nature16946. Nature. 2016. PMID: 26961656 - The Long-Term Relationship between Microbial Metabolism and Greenhouse Gases.
Stein LY. Stein LY. Trends Microbiol. 2020 Jun;28(6):500-511. doi: 10.1016/j.tim.2020.01.006. Epub 2020 Feb 12. Trends Microbiol. 2020. PMID: 32396828 Review. - Microorganisms and climate change: terrestrial feedbacks and mitigation options.
Singh BK, Bardgett RD, Smith P, Reay DS. Singh BK, et al. Nat Rev Microbiol. 2010 Nov;8(11):779-90. doi: 10.1038/nrmicro2439. Nat Rev Microbiol. 2010. PMID: 20948551 Review. - Using meta-omics of contaminated sediments to monitor changes in pathways relevant to climate regulation.
Birrer SC, Dafforn KA, Sun MY, Williams RBH, Potts J, Scanes P, Kelaher BP, Simpson SL, Kjelleberg S, Swarup S, Steinberg P, Johnston EL. Birrer SC, et al. Environ Microbiol. 2019 Jan;21(1):389-401. doi: 10.1111/1462-2920.14470. Epub 2018 Dec 13. Environ Microbiol. 2019. PMID: 30411468 - Microbial nitrous oxide emissions in dryland ecosystems: mechanisms, microbiome and mitigation.
Hu HW, Trivedi P, He JZ, Singh BK. Hu HW, et al. Environ Microbiol. 2017 Dec;19(12):4808-4828. doi: 10.1111/1462-2920.13795. Epub 2017 Jun 22. Environ Microbiol. 2017. PMID: 28493368 Review.
Cited by
- Myxococcus xanthus fruiting body morphology is important for spore recovery after exposure to environmental stress.
Lall D, Glaser MM, Higgs PI. Lall D, et al. Appl Environ Microbiol. 2024 Oct 23;90(10):e0166024. doi: 10.1128/aem.01660-24. Epub 2024 Oct 4. Appl Environ Microbiol. 2024. PMID: 39365039 Free PMC article. - Anoxygenic photo- and chemo-synthesis of phototrophic sulfur bacteria from an alpine meromictic lake.
Di Nezio F, Beney C, Roman S, Danza F, Buetti-Dinh A, Tonolla M, Storelli N. Di Nezio F, et al. FEMS Microbiol Ecol. 2021 Mar 8;97(3):fiab010. doi: 10.1093/femsec/fiab010. FEMS Microbiol Ecol. 2021. PMID: 33512460 Free PMC article. - Cross-kingdom nutrient exchange in the plant-arbuscular mycorrhizal fungus-bacterium continuum.
Duan S, Feng G, Limpens E, Bonfante P, Xie X, Zhang L. Duan S, et al. Nat Rev Microbiol. 2024 Dec;22(12):773-790. doi: 10.1038/s41579-024-01073-7. Epub 2024 Jul 16. Nat Rev Microbiol. 2024. PMID: 39014094 Review. - Microbial functional diversity across biogeochemical provinces in the central Pacific Ocean.
Saunders JK, McIlvin MR, Dupont CL, Kaul D, Moran DM, Horner T, Laperriere SM, Webb EA, Bosak T, Santoro AE, Saito MA. Saunders JK, et al. Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2200014119. doi: 10.1073/pnas.2200014119. Epub 2022 Sep 6. Proc Natl Acad Sci U S A. 2022. PMID: 36067300 Free PMC article. - An ensemble approach to the structure-function problem in microbial communities.
Gopalakrishnappa C, Gowda K, Prabhakara KH, Kuehn S. Gopalakrishnappa C, et al. iScience. 2022 Jan 11;25(2):103761. doi: 10.1016/j.isci.2022.103761. eCollection 2022 Feb 18. iScience. 2022. PMID: 35141504 Free PMC article. Review.
References
- Matsumoto K, Hashioka T, Yamanaka Y. Effect of temperature-dependent organic carbon decay on atmospheric pCO2. J. Geophys. Res. 2007;112:G02007.
- Volk, T. & Hoffert, M. I. In The carbon cycle and atmospheric CO2: natural variations Archean to present. Chapman conference papers, 1984 (eds. Sundquist, E. T. & Broecker, W. S.) 99–110 (American Geophysical Union, 1985).
- Oschlies A, Brandt P, Stramma L, Schmidtko S. Drivers and mechanisms of ocean deoxygenation. Nat. Geosci. 2018;11:467–473. doi: 10.1038/s41561-018-0152-2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources