A Novel, Reliable and Highly Versatile Method to Evaluate Different Prion Decontamination Procedures - PubMed (original) (raw)
A Novel, Reliable and Highly Versatile Method to Evaluate Different Prion Decontamination Procedures
Hasier Eraña et al. Front Bioeng Biotechnol. 2020.
Abstract
Transmissible spongiform encephalopathies (TSEs) are a group of invariably fatal neurodegenerative disorders. The causal agent is an aberrantly folded isoform (PrPSc or prion) of the endogenous prion protein (PrPC) which is neurotoxic and amyloidogenic and induces misfolding of its physiological counterpart. The intrinsic physical characteristics of these infectious proteinaceous pathogens makes them highly resistant to the vast majority of physicochemical decontamination procedures used typically for standard disinfection. This means prions are highly persistent in contaminated tissues, the environment (surfaces) and, of great concern, on medical and surgical instruments. Traditionally, decontamination procedures for prions are tested on natural isolates coming from the brain of infected individuals with an associated high heterogeneity resulting in highly variable results. Using our novel ability to produce highly infectious recombinant prions in vitro we adapted the system to enable recovery of infectious prions from contaminated materials. This method is easy to perform and, importantly, results in highly reproducible propagation in vitro. It exploits the adherence of infectious prion protein to beads of different materials allowing accurate and repeatable assessment of the efficacy of disinfectants of differing physicochemical natures to eliminate infectious prions. This method is technically easy, requires only a small shaker and a standard biochemical technique and could be performed in any laboratory.
Keywords: PMSA; decontamination; in vitro propagation; prion; transmissible spongiform encephalopathy.
Copyright © 2020 Eraña, Pérez-Castro, García-Martínez, Charco, López-Moreno, Díaz-Dominguez, Barrio, González-Miranda and Castilla.
Figures
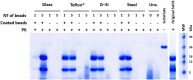
FIGURE 1
Efficient recombinant prion propagation by PMSA using different prion-coated beads as seed. Electrophoresis and total protein staining of the PMSA products obtained, using either 1 or 5 recombinant prion-coated beads of different materials as seeds, showing rec-PrPres after treatment with Proteinase K (PK). Prion-coated beads of glass, zirconia/silica, stainless steel and Teflon® were used as seeds and the same beads without prion coating were also submitted to the same process as controls of cross-contamination or spontaneous rec-PrPres formation. Unseeded (Uns.) reactions without beads and without any seeding were also used for this purpose. All prion-coated beads were able to give rise to rec-PrPres, showing the same electrophoretic patterns as the original Sst01 strain with predominant ∼16 and ∼9 kDa fragments. No cross-contamination or spontaneous formation of rec-PrPres was detected in any of the unseeded reactions either with or without beads. A sample of the substrate rec-PrP without PK digestion is also shown as reference for the size of the undigested rec-PrP. MW, Molecular weight marker.
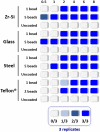
FIGURE 2
Representation of the propagation capacity of each type of prion-coated bead at different PMSA reaction times. In order to characterize the propagation kinetics of each prion-coated bead type and determine the best propagation time window for evaluation of decontamination treatments, either 1 or 5 prion-coated beads were used as seeds (except for Teflon® beads for which 1 or 2 beads were used) and triplicate samples submitted to PMSA from 30 min to 8 h. The maximum number of uncoated beads of each type were also used in the same conditions as controls for cross contamination or spontaneous rec-PrPres generation. The figure represents with different shades of blue the number of replicate tubes in which rec-PrPres was detected for each PMSA time as indicated in the legend. Rec-PrPres was detected for zirconia-silica (Zr-Si) and glass beads as early as 30 min when using 5 beads while the prion-coated steel beads required 1 h and the 2 prion-coated Teflon® beads 2 h. When a single bead of each type was used 3 out of 3 replicates showed detectable rec-PrPres at 2 h except for the Teflon® bead that required 4 h to reach the same result.
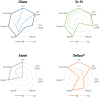
FIGURE 3
Graphical representation of the results obtained for rec-PrPres propagation in PMSA from the different prion-coated beads treated with the chosen different decontamination procedures. Radar charts represent the number of replicate tubes subjected to PMSA showing rec-PrPres by electrophoresis and total protein staining. Colored lines represent the presence of rec-PrPres, which indicates incomplete inactivation of the rec-PrPres adsorbed to the surface of the beads. t2 (dotted lines) refers to the shortest PMSA reaction time in which rec-PrPres was detected in the absence of any decontamination treatment but propagation has not reach a plateau, and t3 (solid lines) refers to the shortest PMSA reaction time in which rec-PrPres signal reached plateau which is considered to be the maximum propagation limit. Each axes of the radar chart represent one of the decontamination treatment used that are detailed in Table 1 (autoclave at 121°C, at 134°C, NaOH, bleach, VirkonTM and AcO-SDS treatment). The plots of the results of Table 2, show clearly that prions adsorbed to steel beads are the ones more easily eliminated by most of the treatments used, and also that bleach and autoclave at 134°C are the most effective for decontamination of prions from most of the surface materials tested, with the exception of Teflon® beads highly susceptible to the treatment with VirkonTM.
FIGURE 4
Representative decontamination treatment evaluation experiments showing examples of a non-efficient, partially efficient and completely efficient treatment for prion decontamination. Electrophoresis and total protein staining of the Proteinase K (PK)-digested PMSA products of three representative experiments are shown as examples of decontamination treatment evaluation by PMSA. Three replicates (R1, R2, and R3) of either 1 or 5 prion-coated zirconia-silica (Zr-Si) beads treated by autoclaving at 121°C for 20 min and submitted to a PMSA round at three time points (t1, t2, and t3), serve as an example of a non-effective inactivation treatment, since rec-PrPres with the characteristic ∼16 kDa predominant band could be detected in all replicates at t2 and t3. Samples at t1 were negative which, being a time point chosen as a control of the process, indicates that the propagation kinetics were as expected. Positive control samples (Control), performed with untreated prion-coated beads of the same type at t1 and t3 demonstrate that the PMSA allowed propagation as expected, and negative controls (Neg C.), performed using non-coated beads of the same time show there was no cross contamination or spontaneous generation during the process. A sample of substrate, not digested with PK, was also included to facilitate size comparisons. As an example of a partially effective treatment, the experiment performed using Teflon® beads treated with 1N NaOH for 1 h is shown. Finally, an example of a completely effective inactivation, the experiment carried out with prion-coated steel beads treated with commercial bleach for 1 h is shown. MW, Molecular weight marker.
Similar articles
- In Vitro Approach To Identify Key Amino Acids in Low Susceptibility of Rabbit Prion Protein to Misfolding.
Eraña H, Fernández-Borges N, Elezgarai SR, Harrathi C, Charco JM, Chianini F, Dagleish MP, Ortega G, Millet Ó, Castilla J. Eraña H, et al. J Virol. 2017 Nov 30;91(24):e01543-17. doi: 10.1128/JVI.01543-17. Print 2017 Dec 15. J Virol. 2017. PMID: 28978705 Free PMC article. - Photocatalytic degradation of prions using the photo-Fenton reagent.
Paspaltsis I, Berberidou C, Poulios I, Sklaviadis T. Paspaltsis I, et al. J Hosp Infect. 2009 Feb;71(2):149-56. doi: 10.1016/j.jhin.2008.09.015. Epub 2008 Nov 17. J Hosp Infect. 2009. PMID: 19013681 - Molecular dynamics studies on the NMR and X-ray structures of rabbit prion proteins.
Zhang J, Zhang Y. Zhang J, et al. J Theor Biol. 2014 Feb 7;342:70-82. doi: 10.1016/j.jtbi.2013.10.005. Epub 2013 Oct 31. J Theor Biol. 2014. PMID: 24184221 - Protein misfolding cyclic amplification (PMCA): Current status and future directions.
Saá P, Cervenakova L. Saá P, et al. Virus Res. 2015 Sep 2;207:47-61. doi: 10.1016/j.virusres.2014.11.007. Epub 2014 Nov 13. Virus Res. 2015. PMID: 25445341 Review. - Transmission and Replication of Prions.
Marín-Moreno A, Fernández-Borges N, Espinosa JC, Andréoletti O, Torres JM. Marín-Moreno A, et al. Prog Mol Biol Transl Sci. 2017;150:181-201. doi: 10.1016/bs.pmbts.2017.06.014. Epub 2017 Aug 7. Prog Mol Biol Transl Sci. 2017. PMID: 28838661 Review.
Cited by
- Advancing surgical instrument safety: A screen of oxidative and alkaline prion decontaminants using real-time quaking-induced conversion with prion-coated steel beads as surgical instrument mimetic.
Heinzer D, Avar M, Pfammatter M, Moos R, Schwarz P, Buhmann MT, Kuhn B, Mauerhofer S, Rosenberg U, Aguzzi A, Hornemann S. Heinzer D, et al. PLoS One. 2024 Jun 13;19(6):e0304603. doi: 10.1371/journal.pone.0304603. eCollection 2024. PLoS One. 2024. PMID: 38870196 Free PMC article. - Understanding the key features of the spontaneous formation of bona fide prions through a novel methodology that enables their swift and consistent generation.
Eraña H, Díaz-Domínguez CM, Charco JM, Vidal E, González-Miranda E, Pérez-Castro MA, Piñeiro P, López-Moreno R, Sampedro-Torres-Quevedo C, Fernández-Veiga L, Tasis-Galarza J, Lorenzo NL, Santini-Santiago A, Lázaro M, García-Martínez S, Gonçalves-Anjo N, San-Juan-Ansoleaga M, Galarza-Ahumada J, Fernández-Muñoz E, Giler S, Valle M, Telling GC, Geijó M, Requena JR, Castilla J. Eraña H, et al. Acta Neuropathol Commun. 2023 Sep 7;11(1):145. doi: 10.1186/s40478-023-01640-8. Acta Neuropathol Commun. 2023. PMID: 37679832 Free PMC article. - Inactivation of Prions by Low-Temperature Sterilization Technology Using Vaporized Gas Derived from a Hydrogen Peroxide-Peracetic Acid Mixture.
Sakudo A, Anraku D, Itarashiki T. Sakudo A, et al. Pathogens. 2020 Dec 31;10(1):24. doi: 10.3390/pathogens10010024. Pathogens. 2020. PMID: 33396428 Free PMC article.
References
- Belondrade M., Nicot S., Beringue V., Coste J., Lehmann S., Bougard D. (2016). Rapid and highly sensitive detection of variant creutzfeldt-jakob disease abnormal prion protein on steel surfaces by protein misfolding cyclic amplification: application to prion decontamination studies. PLoS One 11:e0146833. 10.1371/journal.pone.0146833 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials