Prevalence of readily detected amyloid blood clots in 'unclotted' Type 2 Diabetes Mellitus and COVID-19 plasma: a preliminary report - PubMed (original) (raw)
Prevalence of readily detected amyloid blood clots in 'unclotted' Type 2 Diabetes Mellitus and COVID-19 plasma: a preliminary report
Etheresia Pretorius et al. Cardiovasc Diabetol. 2020.
Abstract
Background: Type 2 Diabetes Mellitus (T2DM) is a well-known comorbidity to COVID-19 and coagulopathies are a common accompaniment to both T2DM and COVID-19. In addition, patients with COVID-19 are known to develop micro-clots within the lungs. The rapid detection of COVID-19 uses genotypic testing for the presence of SARS-Cov-2 virus in nasopharyngeal swabs, but it can have a poor sensitivity. A rapid, host-based physiological test that indicated clotting severity and the extent of clotting pathologies in the individual who was infected or not would be highly desirable.
Methods: Platelet poor plasma (PPP) was collected and frozen. On the day of analysis, PPP samples were thawed and analysed. We show here that microclots can be detected in the native plasma of twenty COVID-19, as well as ten T2DM patients, without the addition of any clotting agent, and in particular that such clots are amyloid in nature as judged by a standard fluorogenic stain. Results were compared to ten healthy age-matched individuals.
Results: In COVID-19 plasma these microclots are significantly increased when compared to the levels in T2DM.
Conclusions: This fluorogenic test may provide a rapid and convenient test with 100% sensitivity (P < 0.0001) and is consistent with the recognition that the early detection and prevention of such clotting can have an important role in therapy.
Keywords: Amyloid; COVID-19; Coagulopathies; Pathologies.
Conflict of interest statement
The authors have no competing interests to declare.
Figures
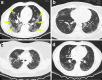
Fig. 1
a–d Representative CT scans of a COVID-19 patient. Yellow arrows show ground glass opacities
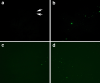
Fig. 2
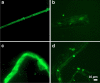
a–d Representative fluorescence micrographs of platelet poor plasma from healthy individuals. Most signals are very weak, as shown by the arrows in a
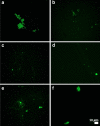
Fig. 3
a–f Representative fluorescence micrographs of platelet poor plasma from Type 2 Diabetes Mellitus (T2DM) patients
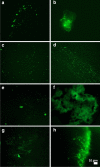
Fig. 4
a–h Representative fluorescence micrographs of platelet poor plasma from COVID-19 patients
Fig. 5
Fibrous or cellular deposits in the plasma smears from COVID-19 patients
Fig. 6
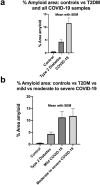
a, b Amyloid % area in platelet poor plasma smears with mean and SEM (p = < 0.0001). a All controls, Type 2 Diabetes Mellitus (T2DM) and all COVID-19 patients. b All controls vs T2DM vs 10 mild and 10 moderate to severely ill COVID-19 patients
Similar articles
- Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC).
Pretorius E, Venter C, Laubscher GJ, Kotze MJ, Oladejo SO, Watson LR, Rajaratnam K, Watson BW, Kell DB. Pretorius E, et al. Cardiovasc Diabetol. 2022 Aug 6;21(1):148. doi: 10.1186/s12933-022-01579-5. Cardiovasc Diabetol. 2022. PMID: 35933347 Free PMC article. - A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications.
Kell DB, Laubscher GJ, Pretorius E. Kell DB, et al. Biochem J. 2022 Feb 17;479(4):537-559. doi: 10.1042/BCJ20220016. Biochem J. 2022. PMID: 35195253 Free PMC article. Review. - Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin.
Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, Kell DB. Pretorius E, et al. Cardiovasc Diabetol. 2021 Aug 23;20(1):172. doi: 10.1186/s12933-021-01359-7. Cardiovasc Diabetol. 2021. PMID: 34425843 Free PMC article. - Impaired anti-SARS-CoV-2 antibody response in non-severe COVID-19 patients with diabetes mellitus: A preliminary report.
Pal R, Sachdeva N, Mukherjee S, Suri V, Zohmangaihi D, Ram S, Puri GD, Bhalla A, Soni SL, Pandey N, Bhansali A, Bhadada SK. Pal R, et al. Diabetes Metab Syndr. 2021 Jan-Feb;15(1):193-196. doi: 10.1016/j.dsx.2020.12.035. Epub 2020 Dec 25. Diabetes Metab Syndr. 2021. PMID: 33385765 Free PMC article. - A Perspective on How Fibrinaloid Microclots and Platelet Pathology May be Applied in Clinical Investigations.
Pretorius E, Kell DB. Pretorius E, et al. Semin Thromb Hemost. 2024 Jun;50(4):537-551. doi: 10.1055/s-0043-1774796. Epub 2023 Sep 25. Semin Thromb Hemost. 2024. PMID: 37748515 Free PMC article. Review.
Cited by
- Proteomic Evidence for Amyloidogenic Cross-Seeding in Fibrinaloid Microclots.
Kell DB, Pretorius E. Kell DB, et al. Int J Mol Sci. 2024 Oct 8;25(19):10809. doi: 10.3390/ijms251910809. Int J Mol Sci. 2024. PMID: 39409138 Free PMC article. - Fibrinaloid Microclots and Atrial Fibrillation.
Kell DB, Lip GYH, Pretorius E. Kell DB, et al. Biomedicines. 2024 Apr 17;12(4):891. doi: 10.3390/biomedicines12040891. Biomedicines. 2024. PMID: 38672245 Free PMC article. Review. - A Blood Supply Pathophysiological Microcirculatory Mechanism for Long COVID.
Koutsiaris AG. Koutsiaris AG. Life (Basel). 2024 Aug 28;14(9):1076. doi: 10.3390/life14091076. Life (Basel). 2024. PMID: 39337860 Free PMC article. - Metabolic Influences Modulating Erythrocyte Deformability and Eryptosis.
Brun JF, Varlet-Marie E, Myzia J, Raynaud de Mauverger E, Pretorius E. Brun JF, et al. Metabolites. 2021 Dec 21;12(1):4. doi: 10.3390/metabo12010004. Metabolites. 2021. PMID: 35050126 Free PMC article. Review. - Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms.
Proal AD, VanElzakker MB. Proal AD, et al. Front Microbiol. 2021 Jun 23;12:698169. doi: 10.3389/fmicb.2021.698169. eCollection 2021. Front Microbiol. 2021. PMID: 34248921 Free PMC article. Review.
References
- Watson J, Whiting PF, Brush JE. Interpreting a covid-19 test result. BMJ. 2020;369:m1808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous