Sleep Disturbance during Infection Compromises Tfh Differentiation and Impacts Host Immunity - PubMed (original) (raw)
Sleep Disturbance during Infection Compromises Tfh Differentiation and Impacts Host Immunity
Edgar Ruz Fernandes et al. iScience. 2020.
Abstract
Although the influence of sleep quality on the immune system is well documented, the mechanisms behind its impact on natural host immunity remain unclear. Meanwhile, it has been suggested that neuroimmune interactions play an important role in this phenomenon. To evaluate the impact of stress-induced sleep disturbance on host immunity, we used a murine model of rapid eye movement sleep deprivation (RSD) integrated with a model of malaria blood-stage infection. We demonstrate that sleep disturbance compromises the differentiation of T follicular helper cells, increasing host susceptibility to the parasite. Chemical inhibition of glucocorticoid (Glcs) synthesis showed that abnormal Glcs production compromised the transcription of Tfh-associated genes resulting in impaired germinal center formation and humoral immune response. Our data demonstrate that RSD-induced abnormal activation of the hypothalamic-pituitary-adrenal axis drives host susceptibility to infection. Understanding the impact of sleep quality in natural resistance to infection may provide insights for disease management.
Keywords: Behavioral Neuroscience; Immunology.
© 2020.
Conflict of interest statement
The authors declare no competing interests.
Figures
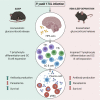
Graphical abstract
Figure 1
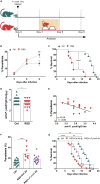
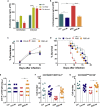
REM Sleep Deprivation Impairs the Control of Malaria Infection by Reducing Parasite-Specific Antibody Titers (A) Experimental setup (see also Figure S1). Mice were submitted to REM sleep deprivation (RSD) 3 days after infection with Plasmodium yoelii, while the control group (Ctrl) was kept under regular sleep conditions. (B) Parasitemia in Ctrl and RSD mice. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by two-way ANOVA. (C) Mice survival in Ctrl and RSD groups. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗∗p < 0.0001) by log-rank (Mantel-Cox) test. (D) Anti-P. yoelii IgG serum titers in Ctrl and RSD mice at day nine post infection. Data are from six independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test. (E) Correlation between parasitemia and anti-P. yoelii IgG titers. Data are from three independent experiments with at least five mice in each group. (F) Six days after infection, RSD mice were transferred with purified IgGs obtained from control or _P. yoelii_-infected HS animals. Three days later, parasitemia levels were evaluated. Data are from two independent experiments with ten mice in each group. Mean ± SEM (∗∗∗p < 0.001) by one-way ANOVA. (G) Mice survival after passive immunization with purified IgG from control or _P. yoelii_-infected animals. Data are from two independent experiments with ten mice in each group. Mean ± SEM (∗∗p < 0.01, ∗∗∗∗p < 0.0001) by log-rank (Mantel-Cox) test.
Figure 2
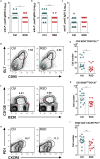
REM Sleep Deprivation Impairs Antibody Production and Differentiation of Germinal Center B Cells, Plasma B Cells, and Tfh Cells Infected Ctrl and RSD mice were analyzed at day nine post infection. (A) Anti-P. yoelii IgG2b serum titers in Ctrl and RSD mice. Data are from six independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test. (B) Anti-P. yoelii IgG2c serum titers in Ctrl and RSD mice. Data are from six independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test. (C) Anti-P. yoelii IgG1 serum titers in Ctrl and RSD mice. Data are from six independent experiments with at least five mice in each group. Mean ± SEM. (D) Representative flow cytometry analysis of splenic GC B cells (CD3-B220+GL7+CD95+) in Ctrl and RSD mice. (E) Absolute numbers of splenic GC B cells (CD3-B220+GL7+CD95+) in Ctrl and RSD mice. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test. (F) Representative flow cytometry analysis of splenic plasma B cells (CD3-B220lowCD138+). (G) Absolute numbers of splenic plasma B cells (CD3-B220lowCD138+) in Ctrl and RSD mice. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test. (H) Representative flow cytometry analysis of splenic Tfh cells (B220−CD3+PD-1+CXCR5+). (I) Absolute numbers of splenic Tfh cells (B220−CD3+PD-1+CXCR5+) in Ctrl and RSD mice. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test.
Figure 3
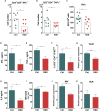
REM Sleep Deprivation Impairs T Cell Activity and Inhibits the Transcription of Genes Associated with Tfh Differentiation Infected Ctrl and RSD mice were analyzed at day nine post infection. (A) Ex vivo analysis of splenic IFN-γ-producing CD3+CD4+ T cells from Ctrl and RSD mice. Data represent one experiment with at least 7 mice per group. Mean ± SD (∗p < 0.05) by two-tailed unpaired t test. (B) Ex vivo analysis of splenic TNF-α-producing CD3+CD4+ T cells from Ctrl and RSD mice. Data represent one experiment with at least 7 mice per group. Mean ± SD (∗p < 0.05) by two-tailed unpaired t test. (C) ELISPOT analysis of IFN-γ-producing cells from Ctrl and RSD mice, after culture with recombinant P. yoelii MSP119. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test. (D) Levels of IFN-γ in culture supernatants from Ctrl and RSD splenocytes after culture with recombinant P. yoelii MSP-119 for 48h. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test. (E) Levels of TNF-α in culture supernatants from Ctrl and RSD splenocytes after culture with recombinant P. yoelii MSP-119 for 48 hr. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test. (F) Frequency of specific proliferating (CFSElow) CD3+CD4+ T cells after culture in the presence of recombinant P. yoelii MSP-119. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test. (G) Tbx21 mRNA expression by sorted splenic CD4+ T cells from Ctrl and RSD mice. Data are from two independent experiments with at least four mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test. (H) Levels of IL-6 in culture supernatants from Ctrl and RSD splenocytes after culture with recombinant P. yoelii MSP-119 for 48hr. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test. (I) Median fluorescence intensity (MFI) of ICOS on splenic CD3+CD4+ T cells from Ctrl and RSD mice. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by two-tailed unpaired t test. (J) Maf mRNA expression by sorted CD4+ T cells from Ctrl and RSD mice. Data are from two independent experiments with at least four mice in each group. Mean ± SEM (∗∗p < 0.01) by two-tailed unpaired t test. (K) Bcl6 mRNA expression by sorted CD4+ T cells from Ctrl and RSD mice. Data are from two independent experiments with at least four mice in each group. Mean ± SEM.
Figure 4
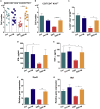
Exacerbated Synthesis of Glucocorticoids Triggered by REM Sleep Deprivation and P. Yoelii Infection Inhibits GC Formation and Host Humoral Immunity Inhibition of corticosterone synthesis was accomplished by treating mice with metyrapone during the RSD period (RSD + M). As an internal control of the drug effect on disease outcome, infected Ctrl received the same treatment (Ctrl + M). (A) Corticosterone concentration in uninfected and infected mice immediately after RSD and after 3 days of sleep recovery (3d rebound). Data of uninfected Ctrl and uninfected RSD mice represent one experiment with at least 5 mice in each group. Mean ± SD; data from Ctrl and RSD infected mice are from two independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗∗p < 0.0001) by two-way ANOVA. (B) Corticosterone plasma levels in infected Ctrl and RSD mice after metyrapone treatment. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05; ∗∗p < 0.01; ∗∗∗∗p < 0.0001) by one-way ANOVA. (C) Parasitemia levels in Ctrl and RSD mice treated or not with metyrapone. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗∗p < 0.0001) by two-way ANOVA. (D) Mice survival following treatment with metyrapone. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by log-rank (Mantel-Cox) test. (E) Anti-P. yoelii IgG1 serum titers in Ctrl and RSD mice treated or not with metyrapone. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by one-way ANOVA. (F) Absolute numbers of splenic GC B cells (CD3-B220+GL7+CD95+) in Ctrl and RSD mice treated or not with metyrapone. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗p < 0.001) by one-way ANOVA. (G) Absolute numbers of splenic plasma B cells (CD3-B220lowCD138+) in Ctrl and RSD mice treated or not with metyrapone. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by one-way ANOVA.
Figure 5
Exacerbated Synthesis of Glucocorticoids Triggered by REM Sleep Deprivation and P. Yoelii Infection Inhibits Tfh Cell Differentiation Inhibition of corticosterone synthesis was accomplished by treating mice with metyrapone during the RSD period (RSD + M). Analyses were performed at day nine post infection. As an internal control of the drug effect on malaria outcome, infected Ctrl received the same treatment (Ctrl + M). (A) Absolute numbers of splenic Tfh cells (B220−CD3+PD-1+CXCR5+) in Ctrl and RSD mice treated or not with metyrapone. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗∗p < 0.0001) by one-way ANOVA. (B) Ex vivo analysis of proliferating CD4+ T cells stained with Ki67+. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗∗∗∗p < 0.0001). (C) Frequency of specific proliferating (CFSElow) CD3+CD4+ T cells after culture in the presence of recombinant P. yoelii MSP-119 for five days. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by one-way ANOVA. (D) Levels of IFN-γ in cell supernatants from Ctrl and RSD splenocytes after culture with recombinant P. yoelii MSP-119 for 48 hr. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by one-way ANOVA. (E) Levels of TNF-α in cell supernatants from Ctrl and RSD splenocytes after culture with recombinant P. yoelii MSP-119 for 48 hr. Data are from three independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by one-way ANOVA. (F) Tbx21 mRNA expression by sorted CD4+ T cells from Ctrl and RSD mice. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗∗p < 0.01) by one-way ANOVA. (G) Maf mRNA expression by sorted CD4+ T cells from Ctrl and RSD mice. Data are from two independent experiments with at least five mice in each group. Mean ± SEM (∗p < 0.05) by one-way ANOVA.
Similar articles
- IFNAR1-Signalling Obstructs ICOS-mediated Humoral Immunity during Non-lethal Blood-Stage Plasmodium Infection.
Sebina I, James KR, Soon MS, Fogg LG, Best SE, Labastida Rivera F, Montes de Oca M, Amante FH, Thomas BS, Beattie L, Souza-Fonseca-Guimaraes F, Smyth MJ, Hertzog PJ, Hill GR, Hutloff A, Engwerda CR, Haque A. Sebina I, et al. PLoS Pathog. 2016 Nov 3;12(11):e1005999. doi: 10.1371/journal.ppat.1005999. eCollection 2016 Nov. PLoS Pathog. 2016. PMID: 27812214 Free PMC article. - Persistent Simian Immunodeficiency Virus Infection Drives Differentiation, Aberrant Accumulation, and Latent Infection of Germinal Center Follicular T Helper Cells.
Xu H, Wang X, Malam N, Aye PP, Alvarez X, Lackner AA, Veazey RS. Xu H, et al. J Virol. 2015 Nov 25;90(3):1578-87. doi: 10.1128/JVI.02471-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26608323 Free PMC article. - Early T Follicular Helper Cell Responses and Germinal Center Reactions Are Associated with Viremia Control in Immunized Rhesus Macaques.
Helmold Hait S, Vargas-Inchaustegui DA, Musich T, Mohanram V, Tuero I, Venzon DJ, Bear J, Rosati M, Vaccari M, Franchini G, Felber BK, Pavlakis GN, Robert-Guroff M. Helmold Hait S, et al. J Virol. 2019 Feb 5;93(4):e01687-18. doi: 10.1128/JVI.01687-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463978 Free PMC article. - Tfh1 Cells in Germinal Centers During Chronic HIV/SIV Infection.
Velu V, Mylvaganam G, Ibegbu C, Amara RR. Velu V, et al. Front Immunol. 2018 Jun 6;9:1272. doi: 10.3389/fimmu.2018.01272. eCollection 2018. Front Immunol. 2018. PMID: 29928280 Free PMC article. Review. - Insights Into the Molecular Mechanisms of T Follicular Helper-Mediated Immunity and Pathology.
Qin L, Waseem TC, Sahoo A, Bieerkehazhi S, Zhou H, Galkina EV, Nurieva R. Qin L, et al. Front Immunol. 2018 Aug 15;9:1884. doi: 10.3389/fimmu.2018.01884. eCollection 2018. Front Immunol. 2018. PMID: 30158933 Free PMC article. Review.
Cited by
- Role of sleep deprivation in immune-related disease risk and outcomes.
Garbarino S, Lanteri P, Bragazzi NL, Magnavita N, Scoditti E. Garbarino S, et al. Commun Biol. 2021 Nov 18;4(1):1304. doi: 10.1038/s42003-021-02825-4. Commun Biol. 2021. PMID: 34795404 Free PMC article. Review. - Sleep duration time and human papillomavirus infection risk: The U-shaped relationship revealed by NHANES data.
Hu H, Wu Y, Zhao M, Liu J, Xie P. Hu H, et al. PLoS One. 2024 Apr 5;19(4):e0301212. doi: 10.1371/journal.pone.0301212. eCollection 2024. PLoS One. 2024. PMID: 38578744 Free PMC article. - Effect of glucocorticoid blockade on inflammatory responses to acute sleep fragmentation in male mice.
Hasan ZW, Nguyen VT, Ashley NT. Hasan ZW, et al. PeerJ. 2024 Jun 28;12:e17539. doi: 10.7717/peerj.17539. eCollection 2024. PeerJ. 2024. PMID: 38952964 Free PMC article. - Help me help you: emerging concepts in T follicular helper cell differentiation, identity, and function.
Wellford SA, Schwartzberg PL. Wellford SA, et al. Curr Opin Immunol. 2024 Apr;87:102421. doi: 10.1016/j.coi.2024.102421. Epub 2024 May 10. Curr Opin Immunol. 2024. PMID: 38733669 Review. - T-follicular helper cells in malaria infection and roles in antibody induction.
Soon MSF, Nalubega M, Boyle MJ. Soon MSF, et al. Oxf Open Immunol. 2021 Apr 10;2(1):iqab008. doi: 10.1093/oxfimm/iqab008. eCollection 2021. Oxf Open Immunol. 2021. PMID: 36845571 Free PMC article. Review.
References
- Adam I., Nour B.Y., Almahi W.A., Omer E.S., Ali N.I. Malaria susceptibility and cortisol levels in pregnant women of eastern Sudan. Int. J. Gynaecol. Obstet. 2007;98:260–261. - PubMed
- Andersen M.L., Martins P.J., D'almeida V., Bignotto M., Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J. Sleep Res. 2005;14:83–90. - PubMed
- Ashley E.A., Pyae Phyo A., Woodrow C.J. Malaria. Lancet. 2018;391:1608–1621. - PubMed
LinkOut - more resources
Full Text Sources