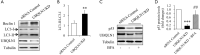The impacts of ubiquilin 1 (UBQLN1) knockdown on cells viability, proliferation, and apoptosis are mediated by p53 in A549 lung cancer cells - PubMed (original) (raw)
The impacts of ubiquilin 1 (UBQLN1) knockdown on cells viability, proliferation, and apoptosis are mediated by p53 in A549 lung cancer cells
Xinghua Zhang et al. J Thorac Dis. 2020 Oct.
Abstract
Background: Little is known about the relationship between ubiquilin 1 (UBQLN1) and p53, both of them have been implicated in the development and progression of non-small cell lung cancer (NSCLC). In this study, we aimed to explore the role of loss of UBQLN1 in cell viability and proliferation, and cell apoptosis in human lung adenocarcinoma A549 cells.
Methods: Cell viability, proliferation, and apoptosis were determined by MTT, BrdU, and TUNEL assays, respectively. Adenoviruses carrying cDNA or siRNA were used to overexpress or silence target protein. Dihydroethidium (DHE) staining was performed to measure the real-time formation of intracellular reactive oxygen species (ROS). The chymotrypsin-like activity of 20S proteasome core was determined by using synthetic fluorogenic peptide substrate.
Results: UBQLN1 silencing led to a reduction of p53 protein levels and overexpression of p53 reversed the effects of UBQLN1 knockdown (KD) on cell viability, proliferation, and apoptosis. Furthermore, deficiency of UBQLN1 activated autophagy activity but did not affect proteasome activity. Inhibition of autophagy restored p53 protein levels in UBQLN1-KD A549 cells. In addition, UBQLN1 KD markedly inhibited phosphorylation of mammalian target of rapamycin (mTOR) and its downstream ribosomal S6 kinase (S6K).
Conclusions: Our experiments suggested that the regulation of UBQLN1 on cell viability, proliferation, and apoptosis was mediated by mTOR/autophagy/p53 signaling pathway.
Keywords: Ubiquilin 1 (UBQLN1); autophagy; mammalian target of rapamycin (mTOR); non-small cell lung cancer (NSCLC); p53.
2020 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd-20-1362). The authors have no conflicts of interest to declare.
Figures
Figure 1
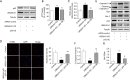
Effects of UBQLN1 knockdown (KD) on cell viability, proliferation, and apoptosis in A549 cells. (A) Cell viability determined by MTT assay; (B) cell proliferation determined by BrdU assay; (C) cell apoptosis determined by TUNEL assay. Left: representative images showing apoptotic cells; right: quantification of cell apoptosis; (D) representative Western blot images showing apoptosis markers; (E) quantification of cleaved caspase-3 in (D); (F) quantification of protein levels of Bcl-2 and Bax in (D); (G) quantification of Bcl-2/Bax in (D). n=4. **, P<0.01 and ***, P<0.001 vs. siRNA control group. UBQLN1, ubiquilin 1; KD, knockdown.
Figure 2
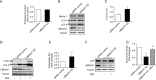
Effects of p53 overexpression on cell viability, proliferation, and apoptosis in UBQLN1-KD A549 cells. (A) Representative Western blot images showing p53 protein levels; (B) cell viability determined by MTT assay; (C) cell proliferation determined by BrdU assay; (D) cell apoptosis determined by TUNEL assay. Left: representative images showing apoptotic cells; right: quantification of cell apoptosis; (E) representative Western blot images showing apoptosis markers; (F) quantification of cleaved caspase-3 in (E); (G) quantification of Bcl-2/Bax in (E). n=4. ***, P<0.001 vs. siRNA control group; ##, P<0.01 and ###, P<0.001 vs. UBQLN1 KD group. OE, overexpression; UBQLN1, ubiquilin 1; KD, knockdown.
Figure 3
Effects of UBQLN1 knockdown (KD) on the activities of proteasome and autophagy in A549 cells. (A) Effects of UBQLN1 KD on proteasome activity; (B) representative Western blot images showing the effects of UBQLN1 KD on autophagic markers; (C) quantification of LC3-II/LC3-I in (B); (D) representative Western blot images showing the effects of autophagy inhibitor BFA on LC3-II expression; (E) quantification of autophagic flux based on (D); (F) representative Western blot images showing the effects of autophagy inhibitor BFA on p53 protein levels; (G) quantification of p53 protein levels in (F). n=4. **, P<0.01 and ***, P<0.001 vs. siRNA control group; ##, P<0.01 vs. UBQLN1 KD group. UBQLN1, ubiquilin 1; BFA, brefeldin A.
Figure 4
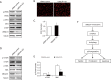
Effects of UBQLN1 knockdown (KD) on ER stress, ROS formation, and the mTOR signaling in A549 cells. (A) Representative Western blot images showing proteins markers of ER stress; (B) representative images showing real time formation of intracellular ROS (magnification ×400); (C) quantification of ROS formation in (B). (D) representative Western blot images showing phosphorylation of mTOR and its downstream S6K; (E) quantification of phosphorylation levels of mTOR and S6K in (C); (F) schematic diagram of UBQLN1 on cell viability, proliferation, and apoptosis in A549 cancer cells. UBQLN1 impairs mTORC1 activity leading to the activation of autophagy and subsequent degradation of p53. Loss of p53 will affect cell viability, proliferation, and apoptosis. n=4. **, P<0.01 and ***, P<0.001 _vs._ siRNA control group; #, P>0.05 vs. siRNA control group. UBQLN1, ubiquilin 1.
Figure S1
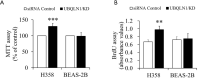
Effects of UBQLN1 knockdown (KD) on cell viability and proliferation in H358 cells and BEAS-2B cells. UBQLN1-silenced and its control cells were grown in RPMI medium containing 10% FBS for 24 h. (A) Cell viability determined by MTT assay; (B) cell proliferation determined by BrdU assay. n=4. **, P<0.01 and ***, P<0.001 vs. siRNA control group. UBQLN1, ubiquilin 1.
Figure S2
Effects of UBQLN1 knockdown (KD) on autophagy and p53 levels in H358 cells. UBQLN1-silenced and its control H358 cells were grown in RPMI medium containing 10% FBS, in the presence or absence of 20 nM BFA for 24 h. (A) Representative Western blot images showing the effects of UBQLN1 KD on autophagic markers; (B) quantification of LC3-II/LC3-I in (A); (C) representative Western blot images showing the effects of autophagy inhibitor BFA on p53 protein levels; (D) quantification of p53 protein levels in (C). n=4. **, P<0.01 and ***, P<0.001 vs. siRNA control group; ##, P<0.01 vs. UBQLN1 KD group. UBQLN1, ubiquilin 1; BFA, brefeldin A.
Similar articles
- Ubiquilin proteins regulate EGFR levels and activity in lung adenocarcinoma cells.
Kurlawala Z, Saurabh K, Dunaway R, Shah PP, Siskind LJ, Beverly LJ. Kurlawala Z, et al. J Cell Biochem. 2021 Jan;122(1):43-52. doi: 10.1002/jcb.29830. Epub 2020 Jul 28. J Cell Biochem. 2021. PMID: 32720736 Free PMC article. - Ubiquilin 1 suppresses the cancer stem cell-like traits of non-small cell lung cancer cells by regulating reactive oxygen species homeostasis.
Liu T, Ma Q, Li W, Hu Y, Yang J, Yao Q. Liu T, et al. Bioengineered. 2021 Dec;12(1):7143-7155. doi: 10.1080/21655979.2021.1979353. Bioengineered. 2021. PMID: 34546848 Free PMC article. - Lycorine Induces Apoptosis of A549 Cells via AMPK-Mammalian Target of Rapamycin (mTOR)-S6K Signaling Pathway.
Zeng H, Fu R, Yan L, Huang J. Zeng H, et al. Med Sci Monit. 2017 Apr 28;23:2035-2041. doi: 10.12659/msm.900742. Med Sci Monit. 2017. PMID: 28450693 Free PMC article. - DRAM1 regulates autophagy and cell proliferation via inhibition of the phosphoinositide 3-kinase-Akt-mTOR-ribosomal protein S6 pathway.
Lu T, Zhu Z, Wu J, She H, Han R, Xu H, Qin ZH. Lu T, et al. Cell Commun Signal. 2019 Mar 22;17(1):28. doi: 10.1186/s12964-019-0341-7. Cell Commun Signal. 2019. PMID: 30902093 Free PMC article.
Cited by
- Humoral immune response to tumor-associated antigen Ubiquilin 1 (UBQLN1) and its tumor-promoting potential in lung cancer.
Wang Y, Ouyang S, Liu M, Si Q, Zhang X, Zhang X, Li J, Wang P, Ye H, Shi J, Song C, Wang K, Dai L. Wang Y, et al. BMC Cancer. 2024 Mar 2;24(1):283. doi: 10.1186/s12885-024-12019-w. BMC Cancer. 2024. PMID: 38431566 Free PMC article. - Association of the Protein-Quality-Control Protein Ubiquilin-1 With Alzheimer's Disease Both in vitro and in vivo.
Zhang C, Inamdar SM, Swaminathan S, Marenda DR, Saunders AJ. Zhang C, et al. Front Neurosci. 2022 Mar 17;16:821059. doi: 10.3389/fnins.2022.821059. eCollection 2022. Front Neurosci. 2022. PMID: 35401099 Free PMC article. - Evaluation and Application of Drug Resistance by Biomarkers in the Clinical Treatment of Liver Cancer.
Huang PS, Wang LY, Wang YW, Tsai MM, Lin TK, Liao CJ, Yeh CT, Lin KH. Huang PS, et al. Cells. 2023 Mar 10;12(6):869. doi: 10.3390/cells12060869. Cells. 2023. PMID: 36980210 Free PMC article. Review. - Abnormally elevated ubiquilin‑1 expression in breast cancer regulates metastasis and stemness via AKT signaling.
Feng X, Cao A, Qin T, Zhang Q, Fan S, Wang B, Song B, Yu X, Li L. Feng X, et al. Oncol Rep. 2021 Nov;46(5):236. doi: 10.3892/or.2021.8187. Epub 2021 Sep 16. Oncol Rep. 2021. PMID: 34528694 Free PMC article. - Breast cancer cell-derived exosome-delivered microRNA-155 targets UBQLN1 in adipocytes and facilitates cancer cachexia-related fat loss.
Sun S, Wang Z, Yao F, Sun K, Li Z, Sun S, Li C. Sun S, et al. Hum Mol Genet. 2023 Jun 19;32(13):2219-2228. doi: 10.1093/hmg/ddad055. Hum Mol Genet. 2023. PMID: 37017334 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous