Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity - PubMed (original) (raw)
Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity
Eun Ae Kang et al. Biomedicines. 2020.
Abstract
The aim of this study was to demonstrate the anti-inflammatory effect of Lactobacillus kefirgranum PRCC-1301-derived extracellular vesicles (PRCC-1301 EVs) on intestinal inflammation and intestinal barrier function. Human intestinal epithelial cells (IECs) Caco-2 were treated with PRCC-1301 EVs and then stimulated with dextran sulfate sodium (DSS). Real-time RT-PCR revealed that PRCC-1301 EVs inhibited the expression of pro-inflammatory cytokines in Caco-2 cells. PRCC-1301 EVs enhanced intestinal barrier function by maintaining intestinal cell integrity and the tight junction. Loss of Zo-1, claudin-1, and occludin in Caco-2 cells and the colitis tissues was recovered after PRCC-1301 EVs treatment, as evidenced by immunofluorescence analysis. Acute murine colitis was induced using 4% DSS and chronic colitis was generated in piroxicam-treated IL-10-/- mice. PRCC-1301 EVs attenuated body weight loss, colon shortening, and histological damage in acute and chronic colitis models in mice. Immunohistochemistry revealed that phosphorylated NF-κB p65 and IκBα were reduced in the colon tissue sections treated with PRCC-1301 EVs. Our results suggest that PRCC-1301 EVs may have an anti-inflammatory effect on colitis by inhibiting the NF-κB pathway and improving intestinal barrier function.
Keywords: Lactobacillus; NF-κB; experimental colitis; extracellular vesicle; tight junction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
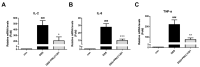
PRCC-1301 extracellular vesicles (EVs) inhibited pro-inflammatory cytokine gene expression in Caco-2 cells. (A) mRNA expression level of (A) IL-2, (B) IL-8, and (C) TNF-α in Caco-2 cells. The results are shown as mean ± SEM. ### p < 0.001 compared with control, * p < 0.05 and ** p < 0.01 and *** p < 0.001 compared with DSS group.
Figure 2
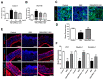
PRCC-1301 EVs recovered increase in intestinal permeability and disruption of tight junction complexes. In vitro permeability assay was performed in (A) Caco-2 and (B) HCT116 cells treated with TNF-α in the absence or presence of PRCC-1301 EVs. The results are shown as mean ± SEM. ### p < 0.001 compared with control, * p < 0.05, ** p < 0.01, and *** p < 0.001 compared with TNF-α-treated group. (C) Expression of ZO-1 in Caco-2 cell monolayers incubated with 2.5% DSS in the absence or presence of PRCC-1301 EVs. (D) Quantification for ZO-1 fluorescence. The results are shown as mean ± SEM. ### p < 0.001 compared with control, ** p < 0.01 compared with DSS group. (E) Expression of ZO-1, claudin-1, and occludin was examined in control, DSS, and PRCC-1301 EV-treated mouse colon tissue. (F) Quantification for ZO-1, claudin-1, and occluding fluorescence, respectively. Nuclei were counterstained with 4, 6-diamidino-2-phenylindole (DAPI). The results are shown as mean ± SEM. ### p < 0.001 compared with control, ** p < 0.01 and *** p < 0.001 compared with DSS group. Scale bar = 100 µm.
Figure 3
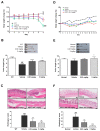
PRCC-1301 EVs prevented DSS-induced acute colitis and attenuated chronic colitis in IL10-/-. (A,D) Body weight, (B,E) colon length, and (C,F) histological evaluation in DSS‑induced and IL10-/- colitis, respectively. The results are shown as mean ± SEM. ### p < 0.001 compared with wild-type (WT), * p < 0.05, ** p < 0.01, and *** p < 0.001 compared with vehicle group. Scale bar = 100 µm.
Figure 4
PRCC-1301 EVs suppressed NF-κB activation in DSS-induced acute colitis. Representative images of Immunohistochemistry (IHC) staining of phospho-NF-κB p65 and phospho-IκBα in 3 mg/kg of PRCC-1301 EV-treated mice. (A,B) The expression of phospho-NF-κB p65 in DSS-induced colitis mice. The arrow indicates phospho-NF-κB p65 positive cells. The expression of phospho-IκBα in (C,D) DSS-induced colitis. The results are shown as mean ± SEM. ### p < 0.001 compared with WT, *** p < 0.001 compared with vehicle group. Scale bar = 100 µm.
Similar articles
- F. prausnitzii-derived extracellular vesicles attenuate experimental colitis by regulating intestinal homeostasis in mice.
Ye L, Wang Y, Xiao F, Wang X, Li X, Cao R, Zhang J, Zhang T. Ye L, et al. Microb Cell Fact. 2023 Nov 15;22(1):235. doi: 10.1186/s12934-023-02243-7. Microb Cell Fact. 2023. PMID: 37968625 Free PMC article. - Effect of toll-like receptor 3 agonist poly I:C on intestinal mucosa and epithelial barrier function in mouse models of acute colitis.
Zhao HW, Yue YH, Han H, Chen XL, Lu YG, Zheng JM, Hou HT, Lang XM, He LL, Hu QL, Dun ZQ. Zhao HW, et al. World J Gastroenterol. 2017 Feb 14;23(6):999-1009. doi: 10.3748/wjg.v23.i6.999. World J Gastroenterol. 2017. PMID: 28246473 Free PMC article. - Effects of 17β-Estradiol on Colonic Permeability and Inflammation in an Azoxymethane/Dextran Sulfate Sodium-Induced Colitis Mouse Model.
Song CH, Kim N, Sohn SH, Lee SM, Nam RH, Na HY, Lee DH, Surh YJ. Song CH, et al. Gut Liver. 2018 Nov 15;12(6):682-693. doi: 10.5009/gnl18221. Gut Liver. 2018. PMID: 30400733 Free PMC article. - Clostridium butyricum protects the epithelial barrier by maintaining tight junction protein expression and regulating microflora in a murine model of dextran sodium sulfate-induced colitis.
Li H, Gong Y, Xie Y, Sun Q, Li Y. Li H, et al. Scand J Gastroenterol. 2018 Sep;53(9):1031-1042. doi: 10.1080/00365521.2016.1192678. Epub 2018 Aug 24. Scand J Gastroenterol. 2018. PMID: 30141701 - Wogonoside alleviates colitis by improving intestinal epithelial barrier function via the MLCK/pMLC2 pathway.
Huang S, Fu Y, Xu B, Liu C, Wang Q, Luo S, Nong F, Wang X, Huang S, Chen J, Zhou L, Luo X. Huang S, et al. Phytomedicine. 2020 Mar;68:153179. doi: 10.1016/j.phymed.2020.153179. Epub 2020 Feb 3. Phytomedicine. 2020. PMID: 32062328
Cited by
- Photodynamic Therapy of LD4-Photosensitizer Attenuates the Acute Pneumonia Induced by Klebsiella pneumoniae.
Tao Z, Li X, Yu H, Wu J, Wen Y, Liu T. Tao Z, et al. ACS Pharmacol Transl Sci. 2024 Mar 5;7(4):1101-1113. doi: 10.1021/acsptsci.3c00392. eCollection 2024 Apr 12. ACS Pharmacol Transl Sci. 2024. PMID: 38633581 - ROS-responsive polymer nanoparticles with enhanced loading of dexamethasone effectively modulate the lung injury microenvironment.
Muhammad W, Zhu J, Zhai Z, Xie J, Zhou J, Feng X, Feng B, Pan Q, Li S, Venkatesan R, Li P, Cao H, Gao C. Muhammad W, et al. Acta Biomater. 2022 Aug;148:258-270. doi: 10.1016/j.actbio.2022.06.024. Epub 2022 Jun 18. Acta Biomater. 2022. PMID: 35724918 Free PMC article. - Kefir and Its Biological Activities.
Azizi NF, Kumar MR, Yeap SK, Abdullah JO, Khalid M, Omar AR, Osman MA, Mortadza SAS, Alitheen NB. Azizi NF, et al. Foods. 2021 May 27;10(6):1210. doi: 10.3390/foods10061210. Foods. 2021. PMID: 34071977 Free PMC article. Review. - Anti-inflammatory effects of extracellular vesicles and cell-free supernatant derived from Lactobacillus crispatus strain RIGLD-1 on Helicobacter pylori-induced inflammatory response in gastric epithelial cells in vitro.
Fakharian F, Sadeghi A, Pouresmaeili F, Soleimani N, Yadegar A. Fakharian F, et al. Folia Microbiol (Praha). 2024 Aug;69(4):927-939. doi: 10.1007/s12223-024-01138-3. Epub 2024 Feb 3. Folia Microbiol (Praha). 2024. PMID: 38308067 - Novel Horizons in Postbiotics: Lactobacillaceae Extracellular Vesicles and Their Applications in Health and Disease.
González-Lozano E, García-García J, Gálvez J, Hidalgo-García L, Rodríguez-Nogales A, Rodríguez-Cabezas ME, Sánchez M. González-Lozano E, et al. Nutrients. 2022 Dec 13;14(24):5296. doi: 10.3390/nu14245296. Nutrients. 2022. PMID: 36558455 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources