Candesartan modulates microglia activation and polarization via NF-κB signaling pathway - PubMed (original) (raw)
Candesartan modulates microglia activation and polarization via NF-κB signaling pathway
Shuyan Qie et al. Int J Immunopathol Pharmacol. 2020 Jan-Dec.
Abstract
Microglia are diverse cells that acquire different functional phenotypes in response to microenvironment in which they reside. Several transcriptional regulators have been identified that regulate different microglia phenotypes. They are mainly stimulated into two opposing phenotypes, classically (M1) and alternatively (M2) phenotype. Regulating microglia polarization from M1 to M2 state has been suggested as a potential therapeutic approach in treatment of CNS disorders. Candesartan, an angiotensin II type I receptors antagonist, exerts beneficial effects for antioxidant, anti-inflammation, neurotrophic, and anti-apoptotic function. However, the effect of candesartan on microglia polarization and underlying mechanisms remain unknown. In this study, the resting microglia were stimulated to M1 microglia with lipopolysaccharide (LPS) and interferon-γ (IFN-γ), and then treated with vehicle or candesartan for 24 h. RT-PCR was utilized to detect the mRNA expression of microglia phenotype markers and inflammatory cytokines. Microglia phenotype markers and toll-like receptor 4 (TLR4)/nuclear factor kappa B (NF-κB) pathway were determined by western blot. A neuron-microglia co-culture system was used to determine whether candesartan could ameliorate the neurotoxic effect of M1 microglia to oxygen-glucose deprivation (OGD) neuron. Candesartan treatment reduced the expression of M1 markers, and increased M2 markers. Meanwhile, candesartan reduced fluorescence intensity and protein level of M1 marker and enhanced M2 marker. Candesartan also regulated the neuroinflammatory response via reducing the release of pro-inflammatory cytokines and increasing anti-inflammatory cytokines in LPS + IFN-γ stimulated BV2 cells. Candesartan markedly inhibited the protein level of TLR4, the phosphorylation of IKBα and p65, and suppressed nuclear translocation of NF-κB p65. BAY 11-7085, a NF-κB inhibitor, remarkably enlarged the inhibitory effect of candesartan on NF-κB pathway. In addition, M1 phenotype microglia exacerbated post-OGD N2a cells death and LDH release, whereas candesartan reversed such neurotoxic effect. Candesartan treatment may ameliorate stroke-induced neuronal damage through shifting microglia to M2 phenotype in a TLR4/NF-κB-dependent manner.
Keywords: BV2 cells; NF-κB; candesartan; microglia polarization; neuroinflammation.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
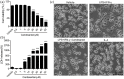
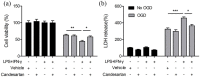
Figure 1.
Effects of candesartan on cell viability and LDH release of BV2 microglial cells. BV2 cells were treated with vehicle or candesartan (0.5, 1, 5, 10, 20, 30, 40, 50 μM) for 24 h. (a) The cell viability was revealed by CCK-8 assay. (b) The cytotoxicity of candesartan on BV2 cells was detected by LDH assay. (c) Images of BV2 cells were taken after treatment with LPS (100 ng/mL) + IFN-γ (20 ng/mL), or IL-4 (20 ng/mL), together with vehicle, candesartan (1 μM) for 24 h. Scale bars represent 50 μm (lower). Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate. ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
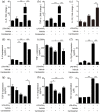
Figure 2.
Candesartan suppresses the mRNA expression of M1 markers and promotes M2 markers in LPS + IFN-γ stimulated BV2 microglia cells. BV2 cells were stimulated with LPS (100 ng/mL) + IFN-γ (20 ng/mL), and then treated with vehicle or candesartan (1 μM) for 24 h. The mRNA expression for M1 markers CD11b (a) and iNOS (b), and M2 markers YM1/2 (c) and CCL22 (d) were examined by RT-PCR. Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate. **P < 0.01, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
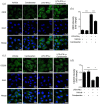
Figure 3.
Candesartan treatment reduces fluorescence intensity of M1 marker and enhances M2 marker in BV2 microglia cells. BV2 cells were stimulated with LPS (100 ng/mL) + IFN-γ (20 ng/mL), and then treated with vehicle or candesartan (1 μM) for 24 h. Representative fluorescence images show M1 marker iNOS (green, a) and M2 marker CD206 (green, c) staining. The nuclei were stained with DAPI (blue) solution. Scale bars = 20 μm. Quantification of the fluorescence intensity of iNOS (b) and CD206 (d) in BV2 cells. Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate. *P < 0.05, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
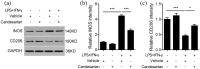
Figure 4.
Candesartan treatment decreases the protein expression of M1 marker and increases M2 marker in BV2 cells. BV2 microglia cells were stimulated with LPS (100 ng/mL) + IFN-γ (20 ng/mL), and then treated with vehicle or candesartan (1 μM) for 24 h. Representative Western blot (a) and quantitative analysis of M1 marker iNOS (b) and M2 marker CD206 (c). GAPDH was used as a loading control. Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate. *P < 0.05, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
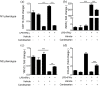
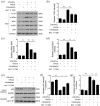
Figure 5.
Candesartan inhibits pro-inflammatory cytokines and enhances anti-inflammatory cytokines in LPS + IFN-γ activated BV2 microglial cells. BV2 cells were cultured with LPS (100 ng/mL) + IFN-γ (20 ng/mL), together with vehicle or candesartan (1 μM) for 24 h. (a–c) The mRNA expressions for pro-inflammatory cytokines (IL-6 and TNF-α) and anti-inflammatory cytokine (IL-10) were examined by RT-PCR. (d–h) The concentration of pro-inflammatory cytokines (IL-6, TNF-α, and IL-12p70) and anti-inflammatory cytokines (TGF-β and IL-10) were examined by ELISA assay. (i) NO assay. Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
Figure 6.
Candesartan shifts microglia polarization via the inhibition of TLR4/NF-κB signaling pathway. BV2 was treated for 30 min with an NF-κB inhibitor (BAY 11-7085, 1 μg/mL), followed by stimulation with LPS (100 ng/mL) + IFN-γ (20 ng/mL) in the presence of vehicle or candesartan (1 μM) for 24 h. Representative western blot (a) and quantification analysis of TLR4 (b), p-IKBα/IKBα (c), and p-p65/p65 (d). (e–g) The protein expressions of p65 in nuclear and cytoplasm were measured by western blot, respectively. Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
Figure 7.
Candesartan reduces the neurotoxic effect of M1 microglia in co-cultured neurons. BV2 cells in transwell were exposed to regular microglia media (resting phenotype), or LPS (100 ng/mL) + IFN-γ (20 ng/mL) (M1 phenotype) for 24 h in the absence or presence of candesartan (1 μM). Neuronal N2a cells were subjected to OGD for 3 h. BV2 cells in transwell was applied over the non-OGD or post-OGD N2a cultures for 24 h. (a) N2a viability was quantified by MTT assay. (b) Cytotoxicity was quantified by LDH release. Data is expressed as mean ± SEM. Samples were collected from three independent experiments, each performed in duplicate. *P < 0.05, **P < 0.01, ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc test.
Similar articles
- Oxymatrine inhibits neuroinflammation byRegulating M1/M2 polarization in N9 microglia through the TLR4/NF-κB pathway.
Wang XL, Chen F, Shi H, Zhang M, Yan L, Pei XY, Peng XD. Wang XL, et al. Int Immunopharmacol. 2021 Nov;100:108139. doi: 10.1016/j.intimp.2021.108139. Epub 2021 Sep 10. Int Immunopharmacol. 2021. PMID: 34517275 - Baicalein ameliorates ischemic brain damage through suppressing proinflammatory microglia polarization via inhibiting the TLR4/NF-κB and STAT1 pathway.
Ran Y, Qie S, Gao F, Ding Z, Yang S, Tian G, Liu Z, Xi J. Ran Y, et al. Brain Res. 2021 Nov 1;1770:147626. doi: 10.1016/j.brainres.2021.147626. Epub 2021 Aug 19. Brain Res. 2021. PMID: 34418356 - Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells.
Zhang J, Zheng Y, Luo Y, Du Y, Zhang X, Fu J. Zhang J, et al. Mol Immunol. 2019 Dec;116:29-37. doi: 10.1016/j.molimm.2019.09.020. Epub 2019 Oct 4. Mol Immunol. 2019. PMID: 31590042 - The natural (poly)phenols as modulators of microglia polarization via TLR4/NF-κB pathway exert anti-inflammatory activity in ischemic stroke.
Li R, Zhou Y, Zhang S, Li J, Zheng Y, Fan X. Li R, et al. Eur J Pharmacol. 2022 Jan 5;914:174660. doi: 10.1016/j.ejphar.2021.174660. Epub 2021 Dec 1. Eur J Pharmacol. 2022. PMID: 34863710 Review. - Ciprofloxacin and levofloxacin attenuate microglia inflammatory response via TLR4/NF-kB pathway.
Zusso M, Lunardi V, Franceschini D, Pagetta A, Lo R, Stifani S, Frigo AC, Giusti P, Moro S. Zusso M, et al. J Neuroinflammation. 2019 Jul 18;16(1):148. doi: 10.1186/s12974-019-1538-9. J Neuroinflammation. 2019. PMID: 31319868 Free PMC article. Review.
Cited by
- Pharmaceutical Potential of Casein-Derived Tripeptide Met-Lys-Pro: Improvement in Cognitive Impairments and Suppression of Inflammation in APP/PS1 Mice.
Matsuzaki Tada A, Hamezah HS, Pahrudin Arrozi A, Abu Bakar ZH, Yanagisawa D, Tooyama I. Matsuzaki Tada A, et al. J Alzheimers Dis. 2022;89(3):835-848. doi: 10.3233/JAD-220192. J Alzheimers Dis. 2022. PMID: 35964178 Free PMC article. - The Implications of Microglial Regulation in Neuroplasticity-Dependent Stroke Recovery.
Qiao C, Liu Z, Qie S. Qiao C, et al. Biomolecules. 2023 Mar 21;13(3):571. doi: 10.3390/biom13030571. Biomolecules. 2023. PMID: 36979506 Free PMC article. Review. - Nanoarchitectonics of tannic acid based injectable hydrogel regulate the microglial phenotype to enhance neuroplasticity for poststroke rehabilitation.
Liu Z, Zhang S, Ran Y, Geng H, Gao F, Tian G, Feng Z, Xi J, Ye L, Su W. Liu Z, et al. Biomater Res. 2023 Oct 31;27(1):108. doi: 10.1186/s40824-023-00444-0. Biomater Res. 2023. PMID: 37908012 Free PMC article. - Microglia Polarization From M1 to M2 in Neurodegenerative Diseases.
Guo S, Wang H, Yin Y. Guo S, et al. Front Aging Neurosci. 2022 Feb 16;14:815347. doi: 10.3389/fnagi.2022.815347. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35250543 Free PMC article. Review.
References
- Keren-Shaul H, Spinrad A, Weiner A, et al. (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169(7): 1276–1290. - PubMed
- Erny D, Prinz M. (2020) How microbiota shape microglial phenotypes and epigenetics. Glia 68(8): 1655–1672. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources