SARS-CoV-2 Receptors and Entry Genes Are Expressed in the Human Olfactory Neuroepithelium and Brain - PubMed (original) (raw)
SARS-CoV-2 Receptors and Entry Genes Are Expressed in the Human Olfactory Neuroepithelium and Brain
Leon Fodoulian et al. iScience. 2020.
Abstract
Reports indicate an association between COVID-19 and anosmia, as well as the presence of SARS-CoV-2 virions in the olfactory bulb. To test whether the olfactory neuroepithelium may represent a target of the virus, we generated RNA-seq libraries from human olfactory neuroepithelia, in which we found substantial expression of the genes coding for the virus receptor angiotensin-converting enzyme-2 (ACE2) and for the virus internalization enhancer TMPRSS2. We analyzed a human olfactory single-cell RNA-seq dataset and determined that sustentacular cells, which maintain the integrity of olfactory sensory neurons, express ACE2 and TMPRSS2. ACE2 protein was highly expressed in a subset of sustentacular cells in human and mouse olfactory tissues. Finally, we found ACE2 transcripts in specific brain cell types, both in mice and humans. Sustentacular cells thus represent a potential entry door for SARS-CoV-2 in a neuronal sensory system that is in direct connection with the brain.
Keywords: Bioinformatics; Biological Sciences; Cell Biology; Microbiology; Omics; Transcriptomics; Virology.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Graphical abstract
Figure 1
ACE2 and TMPRSS2 Expression in Olfactory and Respiratory Nasal Epithelia (A) Schematic of the human nasal respiratory and olfactory epithelia, with their respective cell types. (B) Design of the bulk tissue transcriptome analysis. OE biopsies: n = 3, RE biopsies: n = 3. (C) Volcano plot corresponding to the differential expression analysis, displaying the expression fold change and the associated adjusted p value (∗∗∗∗: p value ≤ 0.0001; ∗: _0.01 <_ p value ≤ 0.05; ns: non-significant, p value > 0.05) between olfactory and respiratory epithelia for each gene. OR genes, olfactory transduction cascade specific genes, and SARS-CoV-2 entry genes ACE2 and TMPRSS2 are highlighted with a color code. (D–G) Transcript quantifications corresponding to (D) OR genes, (E) olfactory sensory epithelium markers and (F and G) SARS-CoV-2 entry proteins in olfactory and respiratory biopsies. A different color was attributed to the data of each patient (n = 4). Olfactory and respiratory epithelium sample data points are shown on a white and gray background, respectively. OE, olfactory epithelium; RE, respiratory epithelium.
Figure 2
Coexpression of ACE2 and TMPRSS2 in Human Sustentacular and Respiratory Ciliated Cells (A) Violin plots displaying ACE2 and TMPRSS2 normalized expression levels in all _ACE2_-positive (n = 169) and _ACE2_-negative (n = 28,557) cells. (B) Visualization of the clustering results reported in Durante et al.(2020) on a uniform manifold approximation and projection (UMAP) plot. “Respiratory columnar cells” was renamed as “Respiratory early secretory cells” based on the expression of SERPINB3. a.u., arbitrary units. (C) Normalized expression levels of ACE2, TMPRSS2, ERMN (a sustentacular cell gene marker), and GSTA2 (a respiratory ciliated cell gene marker), shown on the UMAP plot. norm. UMI, normalized unique molecular identifier counts. (D) ACE2 and TMPRSS2 coexpressing cells, highlighted in black on the UMAP plot. (E) Coexpression of ACE2 and TMPRSS2 in the different cell clusters (n = 26 clusters). Means of ACE2 and TMPRSS2 normalized expression levels are plotted. (F and G) Violin plots displaying ACE2 and TMPRSS2 normalized expression levels in (F) _ACE2_-positive (n = 71) and _ACE2_-negative (n = 3,011) sustentacular cells, and in (G) ACE2 positive (n = 19) and ACE2 negative (n = 567) respiratory ciliated cells. (H) Number of cells per cluster expressing ACE2 and/or TMPRSS2. (I) Percentage of cells per cluster expressing ACE2 and/or TMPRSS2 in all coexpressing clusters (i.e., clusters with double-positive cells). Color gradient scales in (C) and y axis scales in (A), (F) and (G) are in log10.
Figure 3
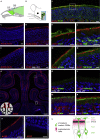
ACE2 expression in the Mouse Olfactory Neuroepithelium (A) Schematic representation of the mouse olfactory system. The sensory tissue and the brain are highlighted in green. The blue frame indicates the coronal section level corresponding to the next panels. OB, olfactory bulb; OE, olfactory epithelium. (B) Schematic representation of a coronal section of the mouse olfactory system. The OE is highlighted in green, and the brain, including the olfactory bulb, in light green. (C–F) Immunostainings on coronal sections of mouse olfactory neuroepithelium with three different anti-ACE2 antibodies (red) and a negative control. (C) ACE2 Ab1, polyclonal antibody against the extracellular domain of mouse ACE2. (D) ACE2 Ab2, polyclonal antibody against the intracellular domain of human ACE2 (that cross reacts with mouse ACE2). (E) ACE2 Ab3, monoclonal antibody against the extracellular domain of human ACE2 (that cross reacts with mouse ACE2). (F) Negative control immunostaining without primary antibody against ACE2. (G) Immunostaining for ACE2 (red) on a coronal section of the mouse olfactory neuroepithelium. Scale bar, 0.2 mm. Squares indicate zones magnified in (H) and (I). The schematic on the lower left indicates that ACE2 expression is observed in the dorsal (H), but not in the ventral part (I) of the olfactory epithelium. (J) Double immunostaining for ACE2 (red) and the neuronal marker TUJ1 (green) in the ACE2-positive part of the mouse olfactory neuroepithelium. The square indicates the portion of the MOE that is magnified in (K)–(R). Scale bar, 20 μm. (K–N) Double immunostaining for ACE2 (red) together with (K) the neuronal marker TUJ1 (green), (L) the marker of mature olfactory sensory neurons OMP (green), (M) the marker for sustentacular cells ERMN (green) and (N) TMPRSS2 (green). (O and P) Negative control immunostaining without primary antibody against (O) ERMN and (P) TMPRSS2. (Q and R) Double immunostaining for the sustentacular cell marker ERMN (red) together with (Q) OMP and (R) TUJ1. (S) Schematic of the external part of the olfactory epithelium, highlighting the relative positions of ACE2, TUJ1, ERMN, and OMP according to the immunostainings shown in (K)–(R). TUJ1 is present in the dendrites, axons, and somata of olfactory sensory neurons, OMP more concentrated in the cell body of olfactory sensory neurons, and ACE2 and ERMN in the apical portion of sustentacular cells. All sections with immunostaining were counterstained with DAPI (blue). When not indicated, scale bars are 10 μm. See also Figures S1–S3.
Figure 4
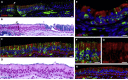
ACE2 Expression in the Human Olfactory Neuroepithelium (A and B) Section of human respiratory epithelium with (A) immunostaining for ERMN (red) and the neuronal marker TUJ1 (green), and (B) the same section stained with Alcian blue and counterstained with nuclear fast red, highlighting a transition between sensory (left) and respiratory (right, note the goblet cells) epithelium. ERMN is expressed in the apical part of sustentacular cells within the olfactory neuroepithelium (arrowheads). (C, E) A portion of human olfactory neuroepithelium immunostained for ACE2 (red) and the neuronal marker TUJ1 (green), and (D) with Alcian blue (note the absence of goblet cells). (F, G) Higher magnification of the external border of the human olfactory neuroepithelium. The same region is shown with (F) and without (G) TUJ1 staining. Nuclei were counterstained with DAPI (blue) or FastRed (red). Scale bars, 10 μm. See also Figures S4 and S5.
Figure 5
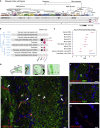
Ace2 and Tmprss2 Expression in the Mouse Brain (A) Mouse brain cell type classification from Zeisel et al. (2018) was visualized via
. Rows with shaded boxes below the tree indicate the gene mean expression levels for each cell type, relative to its maximum. Rbfox3, encoding the neuronal marker NEUN, and Rgs5, a marker of pericytes, are shown in comparison with Ace2 and Tmprss2. (B) Details of the gene expression levels in pericytes (blue edges clade of the classification dendrogram in (A)). Discs represent mean relative expression levels. Maximum values of mean normalized expression levels for Rgs5 and Ace2 are connected to the corresponding circles. (C) Ace2 and Tmprss2 expression levels in the top 10 _Ace2_-expressing cell populations from the
dataset (Saunders et al., 2018). Abbreviations in parentheses denote the origin brain structure. STR, striatum; HC, hippocampus; PC, posterior cortex; FC, frontal cortex; GP, globus pallidus; CB, cerebellum; TH, thalamus; SN, substantia nigra; ENT, entopeduncular. (D) Similar to Figure 3, subsequent panels show coronal sections (blue area) crossing the nasal cavity and the olfactory bulb. As represented in this schematic, olfactory sensory neurons from the olfactory epithelium send axonal projections to the olfactory bulb through the cribriform plate of the ethmoid bone, along the nerve layer. OB, olfactory bulb; OE, olfactory epithelium; nl, nerve layer; gl, glomerular layer; ml, mitral cell layer; gr, granule cell layer. (E–G) Immunostaining for ACE2 (red) and for the neuronal marker TUJ1 (green) on sections of the mouse olfactory bulb. White arrowheads indicate the presence of ACE2 in the pericytes of capillaries. Scale bars, 20 μm. (H) Magnification of capillary ACE2 immunostaining (red). Scale bar, 5 μm. All sections were counterstained with DAPI (blue).
Figure 6
Coexpression of ACE2 and TMPRSS2 in the Human Brain (A) Visualization of the human brain cell-type subclasses on a t-distributed stochastic neighbor embedding (t-SNE) plot. a.u., arbitrary units. (B and C) Normalized expression levels of (B) RBFOX3 (which encodes for the neuronal marker NEUN), (C) ACE2 and TMPRSS2, shown on the t-SNE plot. norm. expr, normalized expression levels. (D) ACE2 and TMPRSS2 coexpressing cells, highlighted in black on the t-SNE plot. (E) Coexpression of ACE2 and TMPRSS2 in the different subclasses (n = 19 subclasses). Means of ACE2 and TMPRSS2 normalized expression levels are plotted. (F) Number of cells per subclass expressing ACE2 and/or TMPRSS2. (G) Percentage of cells per subclass expressing ACE2 and/or TMPRSS2 in all coexpressing subclasses (i.e., subclasses with double-positive cells). Color gradient scales in (B) and (C) are in log10. L, cortical layer; IT, intratelencephalic; CT, corticothalamic; ET, extratelencephalic; NP, near-projecting; LAMP5, lysosomal-associated membrane protein family member 5; PAX6, paired box 6; VIP, vasoactive intestinal polypeptide; SST, somatostatin; PVALB, parvalbumin; OPC, oligodendrocyte precursor cell; VLMC, vascular leptomeningeal cell. See also Figure S6.
Similar articles
- Evidence of SARS-CoV2 Entry Protein ACE2 in the Human Nose and Olfactory Bulb.
Klingenstein M, Klingenstein S, Neckel PH, Mack AF, Wagner AP, Kleger A, Liebau S, Milazzo A. Klingenstein M, et al. Cells Tissues Organs. 2020;209(4-6):155-164. doi: 10.1159/000513040. Epub 2021 Jan 22. Cells Tissues Organs. 2020. PMID: 33486479 Free PMC article. - Supporting Cells of the Human Olfactory Epithelium Co-Express the Lipid Scramblase TMEM16F and ACE2 and May Cause Smell Loss by SARS-CoV-2 Spike-Induced Syncytia.
Hernandez-Clavijo A, Gonzalez-Velandia KY, Rangaswamy U, Guarneri G, Boscolo-Rizzo P, Tofanelli M, Gardenal N, Sanges R, Dibattista M, Tirelli G, Menini A. Hernandez-Clavijo A, et al. Cell Physiol Biochem. 2022 Jun 7;56(3):254-269. doi: 10.33594/000000531. Cell Physiol Biochem. 2022. PMID: 35670331 - Knockout of angiotensin converting enzyme-2 receptor leads to morphological aberrations in rodent olfactory centers and dysfunctions associated with sense of smell.
Mahajan S, Sen D, Sunil A, Srikanth P, Marathe SD, Shaw K, Sahare M, Galande S, Abraham NM. Mahajan S, et al. Front Neurosci. 2023 Jun 19;17:1180868. doi: 10.3389/fnins.2023.1180868. eCollection 2023. Front Neurosci. 2023. PMID: 37404465 Free PMC article. - Pathophysiological relationship between COVID-19 and olfactory dysfunction: A systematic review.
Las Casas Lima MH, Cavalcante ALB, Leão SC. Las Casas Lima MH, et al. Braz J Otorhinolaryngol. 2022 Sep-Oct;88(5):794-802. doi: 10.1016/j.bjorl.2021.04.001. Epub 2021 Apr 25. Braz J Otorhinolaryngol. 2022. PMID: 33965353 Free PMC article. Review. - COVID-19 Anosmia: High Prevalence, Plural Neuropathogenic Mechanisms, and Scarce Neurotropism of SARS-CoV-2?
Liang F, Wang Y. Liang F, et al. Viruses. 2021 Nov 4;13(11):2225. doi: 10.3390/v13112225. Viruses. 2021. PMID: 34835030 Free PMC article. Review.
Cited by
- Lifting the mask on neurological manifestations of COVID-19.
Pezzini A, Padovani A. Pezzini A, et al. Nat Rev Neurol. 2020 Nov;16(11):636-644. doi: 10.1038/s41582-020-0398-3. Epub 2020 Aug 24. Nat Rev Neurol. 2020. PMID: 32839585 Free PMC article. Review. - The need for routine psychiatric assessment of COVID-19 survivors.
Singh OP. Singh OP. Indian J Psychiatry. 2020 Sep-Oct;62(5):457-458. doi: 10.4103/indianjpsychiatry.indianjpsychiatry_1169_20. Epub 2020 Oct 10. Indian J Psychiatry. 2020. PMID: 33678823 Free PMC article. No abstract available. - Psychiatric face of COVID-19.
Steardo L Jr, Steardo L, Verkhratsky A. Steardo L Jr, et al. Transl Psychiatry. 2020 Jul 30;10(1):261. doi: 10.1038/s41398-020-00949-5. Transl Psychiatry. 2020. PMID: 32732883 Free PMC article. Review. - Innate immune signaling in the olfactory epithelium reduces odorant receptor levels: modeling transient smell loss in COVID-19 patients.
Rodriguez S, Cao L, Rickenbacher GT, Benz EG, Magdamo C, Gomez LR, Holbrook EH, Albers AD, Gallagher R, Westover MB, Evans KE, Tatar DJ, Mukerji S, Zafonte R, Boyer EW, Yu CR, Albers MW. Rodriguez S, et al. medRxiv [Preprint]. 2020 Jun 16:2020.06.14.20131128. doi: 10.1101/2020.06.14.20131128. medRxiv. 2020. PMID: 32587994 Free PMC article. Preprint. - Multi-Data Integration Towards a Global Understanding of the Neurological Impact of Human Brain Severe Acute Respiratory Syndrome Coronavirus 2 Infection.
Mesmoudi S, Lapina C, Rodic M, Peschanski D. Mesmoudi S, et al. Front Integr Neurosci. 2022 Jul 13;16:756604. doi: 10.3389/fnint.2022.756604. eCollection 2022. Front Integr Neurosci. 2022. PMID: 35910337 Free PMC article.
References
- Bergstrom U., Giovanetti A., Piras E., Brittebo E.B. Methimazole-induced damage in the olfactory mucosa: effects on ultrastructure and glutathione levels. Toxicol. Pathol. 2003;31:379–387. - PubMed
- Bertram S., Heurich A., Lavender H., Gierer S., Danisch S., Perin P., Lucas J.M., Nelson P.S., Pohlmann S., Soilleux E.J. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS One. 2012;7:e35876. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous