Small molecule inhibition of Dynamin-dependent endocytosis targets multiple niche signals and impairs leukemia stem cells - PubMed (original) (raw)
doi: 10.1038/s41467-020-20091-6.
Sung Kai Chiu 2 3, Jesslyn Saw 2, Hannah McCalmont 4, Veronique Litalien 2, Jacqueline Boyle 2, Stefan E Sonderegger 2, Ngoc Chau 5, Kathryn Evans 4, Loretta Cerruti 2, Jessica M Salmon 2, Adam McCluskey 6, Richard B Lock 4, Phillip J Robinson 5, Stephen M Jane 2 3, David J Curtis 2 3
Affiliations
- PMID: 33277497
- PMCID: PMC7719179
- DOI: 10.1038/s41467-020-20091-6
Small molecule inhibition of Dynamin-dependent endocytosis targets multiple niche signals and impairs leukemia stem cells
Cedric S Tremblay et al. Nat Commun. 2020.
Erratum in
- Author Correction: Small molecule inhibition of Dynamin-dependent endocytosis targets multiple niche signals and impairs leukemia stem cells.
Tremblay CS, Chiu SK, Saw J, McCalmont H, Litalien V, Boyle J, Sonderegger SE, Chau N, Evans K, Cerruti L, Salmon JM, McCluskey A, Lock RB, Robinson PJ, Jane SM, Curtis DJ. Tremblay CS, et al. Nat Commun. 2021 Feb 19;12(1):1288. doi: 10.1038/s41467-021-21688-1. Nat Commun. 2021. PMID: 33608527 Free PMC article. No abstract available.
Abstract
Intensive chemotherapy for acute leukemia can usually induce complete remission, but fails in many patients to eradicate the leukemia stem cells responsible for relapse. There is accumulating evidence that these relapse-inducing cells are maintained and protected by signals provided by the microenvironment. Thus, inhibition of niche signals is a proposed strategy to target leukemia stem cells but this requires knowledge of the critical signals and may be subject to compensatory mechanisms. Signals from the niche require receptor-mediated endocytosis, a generic process dependent on the Dynamin family of large GTPases. Here, we show that Dynole 34-2, a potent inhibitor of Dynamin GTPase activity, can block transduction of key signalling pathways and overcome chemoresistance of leukemia stem cells. Our results provide a significant conceptual advance in therapeutic strategies for acute leukemia that may be applicable to other malignancies in which signals from the niche are involved in disease progression and chemoresistance.
Conflict of interest statement
The authors declare no competing interests.
Figures
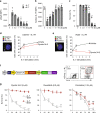
Fig. 1. Dynole 34-2 induces apoptosis by blocking Dynamin-dependent endocytosis in IL-7-dependent Ba/F3 cells.
a–c Levels of phospho-Stat5 (pStat5; a), surface IL-7R (b) and survival (c) of Ba/F3-IL7R cells with increasing doses of Dynole 34-2, assessed by flow cytometry. Mean ± SD from three independent experiments are shown (*P < 0.05, **P < 0.01, ***P < 0.001 compared to vehicle + IL-7). Viability normalized to vehicle + IL-7 control (grey). Baseline is untreated cells without IL-7 (white bar). d, e Representative immunofluorescence staining for IL-7R colocalization with clathrin (d) and the early-endosome marker Rab5 (e) in Ba/F3-IL7R cells stimulated with IL-7. Nuclei were stained using DAPI and colocalization clusters were indicated with a white arrow. Proportion of cells presenting colocalization of IL-7R with either clathrin (d) or Rab5 (e), at different time points following IL-7 stimulation, were determined by reporting the number of cells displaying at least one colocalized staining for green and red fluorescence to the total number of cells, as previously described,. Scale bars: 10 μm. Mean ± SEM from three independent experiments (Supplementary Fig. 1d). Two-way ANOVA test with Sidak’s correction; ***p < 0.001, as compared with Vehicle. f Schematic representation of the TxTCPVIR retroviral vector used for the doxycycline-inducible expression of constitutively active Stat5 (_Stat5_-CA) in Ba/F3-IL7R cells. The inducible expression of the transgene in Ba/F3-IL7RStat5-CA cells can be assessed by measuring levels of mCherry by flow cytometry (right panels). g Cytotoxic activity of Dynole 34-2, Ruxolitinib or Vincristine in IL-7-dependent (+IL-7 −DOX; grey) and IL-7-independent (−IL-7 +DOX; red) Ba/F3-IL7RStat5-CA cells. The doses used correspond to the median inhibitory (IC50) or lethal concentration (LC50) for each drug, determined in Ba/F3-IL7R cells cultured with IL-7 (Fig. 1a–c and Supplementary Fig. 1g, h). Viability is normalized to 100% at the time of initial drug treatment (day 0). Mean ± SD from three independent experiments are shown (***P < 0.001 compared to vehicle +IL-7 −DOX; ###P < 0.001 compared to vehicle −IL-7 +DOX.
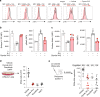
Fig. 2. Dynole 34-2 prevents the activation of the signalling network downstream of IL-7R and Notch1 in pre-leukemic DN3a thymocytes.
a Levels of growth factor-mediated signalling effectors in _Lmo2_Tg DN3a cells treated with vehicle or Dynole 34-2 co-cultured on OP9-DL1 with cytokines, assessed by flow cytometry. Mean fluorescence intensity (MFI) ± SD of n = 4 individual animals analysed in duplicate are shown (Mann–Whitney test; *P < 0.05 and ***P < 0.001 compared to vehicle). b, Internalization assay for IL-7R (left) and downstream activation of Stat5 (pStat5; right) in _Lmo2_Tg DN3a cells in steady state and after stimulation with IL-7, in presence of 0.2 μM Dynole 34-2. MFI ± SD of n = 3 individual mice, performed in duplicate. Student’s _t_-test, **P < 0.01, ***P < 0.001 compared to unstimulated cells. c Surface levels of Notch1 (left) and intracellular expression of Hes1 (right) in _Lmo2_-transgenic DN3a thymocytes, co-cultured overnight on OP9 or OP9-DL1 (DL1) cells in the presence of 0.2 μM Dynole 34-2. MFI ± SD of n = 3 individual animals, performed in duplicate. Student’s _t_-test *P < 0.05, **P < 0.01, ***P < 0.001 compared to OP9 (unstimulated) controls. d, e Absolute numbers of co-cultured _Lmo2_Tg DN3a thymocytes treated in vitro with either vehicle, Dynole 34-2 (0.2 μM), Ruxolitinib (0.2 μM) and DAPT (0.1 μM) for 72 h (d), and in vivo fold expansion of these cells measured 6 weeks post-transplantation in the thymus of recipients (e). Mean ± SD, Student’s _t_-test (N = 4 individual mice). ***P < 0.001 compared to vehicle. Number of recipients engrafted is indicated.
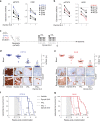
Fig. 3. Inhibition of receptor-mediated endocytosis of IL-7R and Notch1 by Dynole 34-2 impairs pre-LSC self-renewal.
a Treatment schematic and absolute numbers of DN3a T-cell progenitors in the thymus of 6-week-old _Lmo2_Tg mice following treatment with vehicle or Dynole 34-2. Mean ± SEM, Student’s _t_-test; *P < 0.05 compared to vehicle. Each square represents an individual animal. Dashed line indicated the mean absolute number of DN3a thymocytes in wild-type (WT) mice at 6 weeks of age. b Levels of activated Stat5 (pStat5, left) and Hes1 (right) proteins in DN3a cells from _Lmo2_Tg mice treated with vehicle or Dynole 34-2, assessed by flow cytometry. Mean ± SD of n = 3 individual mice are shown. Student’s _t_-test ***P < 0.001 compared to vehicle. c Pre-LSC frequency within the DN3a thymocyte population of _Lmo2_Tg mice treated with vehicle or Dynole 34-2 assessed by limiting dilution assays. Mice were scored positive when T-cell lineage reconstitution was more than 1%, as previously described. Pre-LSC frequencies and [95% confidence intervals] are shown and calculated from three individual mice.
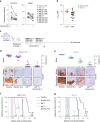
Fig. 4. Dynole 34-2 sensitizes pre-LSCs to induction-like therapy.
a Treatment schematic and absolute numbers of DN3a T-cell progenitors in the thymus of 6-week-old _Lmo2_Tg mice following administration of two rounds of vehicle and Dynole 34-2, alone or combined with VXL chemotherapy. Mean ± SEM, two-way ANOVA with Tukey’s correction test; *P < 0.05 and ***P < 0.001. Each square represents an individual animal. b Representative flow cytometry analysis of the CD4−CD8− (DN) T-cell progenitor populations in the thymus of 6-week-old _Lmo2_Tg mice following administration of two rounds of vehicle and Dynole 34-2, alone or combined with VXL chemotherapy. Mean ± SD (N = 5 individual mice), two-way ANOVA with Bonferroni correction test; *P < 0.05 and ***P < 0.001 compared to vehicle; ###P < 0.001, as compared to VXL, respectively. c Fold expansion of DN3a thymocytes from _Lmo2_Tg mice treated with either vehicle, Dynole 34-2, VXL or combination therapy with Dynole 34-2. Mean ± SEM, two-way ANOVA with Bonferroni correction test *P < 0.05, **P < 0.01 and ***P < 0.001. Each circle represents an individual animal. d Scheme for the intrathymic competition assays in the Mx;YFP and Mx;_YFP;Lmo2_Tg mice, which were injected with poly (I : C) to induce the expression of YFP in the bone marrow stem cells (HSCs), which can be tracked in the thymus 3 weeks post-injection. e Proportion of HSC-derived (%YFP+) DN3a thymocytes in Mx;_YFP;Lmo2_Tg mice treated with vehicle, Dynole 34-2, VXL and the combination Dynole 34-2 + VXL. Mx;YFP mice were used as positive controls. Mean ± SEM (N = 3 individual mice), two-way ANOVA with Tukey’s correction test; *P < 0.05, **P < 0.01 and ***P < 0.001.
Fig. 5. Efficacy of Dynole 34-2 against LSCs from primary _Lmo2_-transgenic T-ALL.
a Flow sorting and schematic representation of the transplantation strategy of purified leukemic populations from three different primary _Lmo2_Tg thymomas. Indicated leukemic populations were purified and 5 × 104 cells were transplanted into sublethally irradiated Cd45.1+ recipients. b Kaplan–Meier curves of mice injected with the purified populations (DN1, n = 5; DN3a, n = 17; DN4, n = 15; ISP8, n = 10; DP, n = 12) from three different _Lmo2_Tg primary leukemias. c Relative levels of activated Stat5 (pStat5) and Hes1 in leukemic DN3a cells from primary _Lmo2_Tg T-ALL treated with Dynole 34-2, after in vitro stimulation of either the IL-7 (left) or Notch1 (right) signalling pathway. Primary leukemias are indicated on the right with T-ALL 249 in blue, T-ALL 582 in green, T-ALL 573 in orange, and other leukemias are in black; unstimulated leukemic DN3a cells treated with vehicle were used as control, and reported as 1 (dashed line). Mean ± SEM (N = 6 primary leukemias, performed in duplicate), two-way ANOVA with Bonferroni correction test; **P < 0.01 as compared to vehicle. d Experimental setting for testing the efficacy of Dynole 34-2, VXL and combination therapy in sublethally irradiated Cd45.1+ recipients injected with _Lmo2_Tg primary leukemias. e, f Relative numbers of leukemic DN3a cells in the thymus, bone marrow, spleen (e) and blood (f) of recipients, 24 h after the last administration of Dynole 34-2, VXL and combination therapy. Absolute numbers of DN3a cells in recipients treated with vehicle were used as control (dashed line) and reported as 100%. Mean ± SEM (N = 3 recipients per individual leukemia, analysed in duplicate), two-way ANOVA with Tukey’s correction test; *P < 0.05, **P < 0.01 and ***P < 0.001 compared to vehicle; ##P < 0.01 and ###P < 0.001 compared to VXL. g Kaplan–Meier curves of sublethally irradiated recipients injected with _Lmo2_Tg primary T-ALL, treated with Dynole 34-2, VXL or combination therapy. Log-rank (Mantel–Cox) test; *P < 0.05 and **P < 0.01 compared to vehicle; #P < 0.05 and ##P < 0.01 compared to VXL. The period of administration is indicated in light grey, with the number of recipients for each cohort indicated for each primary leukemia.
Fig. 6. Dynole 34-2 is effective against human ETP-ALL and mature T-ALL.
a, b Relative levels of IL-7-induced activation of STAT5 (pStat5, left) and NOTCH1-induced expression of HES1 (right) in (a) ETP-ALL and (b) T-ALL cells from human xenografts treated with vehicle and Dynole 34-2, after in vitro stimulation. ETP12 is depicted with solid blue circles, ALL8 with red open circles and all other leukemias in black. Levels in unstimulated xenograft cells treated with vehicle were used as control, and reported as 1 (dashed line). Mean ± SD (N = 6 primary leukemias, performed in duplicate), two-way ANOVA with Bonferroni correction test; *P < 0.05 and **P < 0.01 as compared to vehicle. c Experimental setting for testing the efficacy of Dynole 34-2, VXL and combination therapy in xenograft models of human acute leukemia. Sublethally irradiated NSG recipients were randomized after engraftment was confirmed in the peripheral blood, and subsequently treated when the average proportion of human leukemic cells in the peripheral blood reached 1%. d, e Proportion of patient-derived cells (%hCD45+) in the peripheral blood (top), as well as immunochemistry against hCD45+ leukemic cells in the bone marrow and spleen (bottom) of recipients injected with ETP12 (d) and ALL8 (e), 24 h after the last drug injection. Scale bars: 10 μm. Mean ± SEM, two-way ANOVA with Tukey’s correction test; ***P < 0.0001 compared to vehicle; #P < 0.05 and ###P < 0.0001 compared to VXL. Each circle represents an individual recipient. f, g Kaplan–Meier curves of sublethally irradiated recipients injected with ETP12 (f) and ALL8 (g), administered with either vehicle or Dynole 34-2, as a single agent or in combination with VXL chemotherapy. Log-rank (Mantel–Cox) test; ***P < 0.0001 compared to vehicle; ###P < 0.0001 compared to VXL. The period of administration is indicated in light grey, with the number of recipients for each cohort indicated for ETP12 and ALL8 xenograft models.
Fig. 7. Dynole 34-2 exhibits activity in human AML.
a Relative levels of activated STAT5 (pSTAT5, left) and ERK (pERK, right) in patient-derived AML cells treated with vehicle and Dynole 34-2, after in vitro stimulation with IL-3 (left) and SCF (right). AML01-307 is depicted with solid purple triangles, AML18 with solid turquoise triangles, and all other AML samples in black. Levels in unstimulated xenograft cells treated with vehicle were used as control, and reported as 1 (dashed line). Mean ± SD, two-way ANOVA with Bonferroni correction test; **P < 0.01 and ***P < 0.001 as compared to vehicle (N = 14 primary AML, performed in duplicate). b Median lethal concentration (LC50) of Dynole 34-2 for patient-derived AML cells (N = 22 leukemias), assessed by performing clonogenic assays for 10 days (Supplementary Fig. 9c, d). Human CD34-positive HSPCs (red dashed line; CD34+) from healthy donors (N = 6) were used as controls. Mean ± SEM, two-tailed Mann–Whitney test; **P < 0.01 compared to CD34+ control cells. Each circle represNOTCH1, fraction of leukemic blasts harbouring activating mutations of NOTCH1ents an individual human sample. c, Experimental setting for testing the efficacy of Dynole 34-2, DA5+3 and combination therapy in xenograft models of human AML. Sublethally irradiated NRGS recipients were randomized after engraftment was confirmed in the peripheral blood and subsequently treated when the average proportion of human leukemic cells in the peripheral blood reached 1%. d, e Proportion of patient-derived cells (%hCD45+) in the peripheral blood (top), as well as immunochemistry against hCD45+ leukemic cells in the bone marrow and spleen (bottom) of recipients injected with AML01-307 (d) and AML18 (e), 24 h after the last drug injection. Scale bars: 10 μm. Mean ± SEM, two-way ANOVA with Tukey’s correction test; ***P < 0.0001 compared to vehicle; #P < 0.05 and ###P < 0.0001 compared to VXL. Each triangle represents an individual recipient. f, g Kaplan–Meier curves of sublethally irradiated recipients injected with AML01-307 (f) and AML18 (g), administered with either vehicle or Dynole 34-2, as a single agent or in combination with DA5+3 chemotherapy. Log-rank (Mantel–Cox) test; ***P < 0.0001 compared to vehicle; ###P < 0.0001 compared to DA5+3. The period of administration is indicated in light grey, with the number of recipients for each cohort indicated.
Similar articles
- Targeting RSPO3-LGR4 Signaling for Leukemia Stem Cell Eradication in Acute Myeloid Leukemia.
Salik B, Yi H, Hassan N, Santiappillai N, Vick B, Connerty P, Duly A, Trahair T, Woo AJ, Beck D, Liu T, Spiekermann K, Jeremias I, Wang J, Kavallaris M, Haber M, Norris MD, Liebermann DA, D'Andrea RJ, Murriel C, Wang JY. Salik B, et al. Cancer Cell. 2020 Aug 10;38(2):263-278.e6. doi: 10.1016/j.ccell.2020.05.014. Epub 2020 Jun 18. Cancer Cell. 2020. PMID: 32559496 - In vivo targeting of leukemia stem cells by directing parthenolide-loaded nanoparticles to the bone marrow niche.
Zong H, Sen S, Zhang G, Mu C, Albayati ZF, Gorenstein DG, Liu X, Ferrari M, Crooks PA, Roboz GJ, Shen H, Guzman ML. Zong H, et al. Leukemia. 2016 Jul;30(7):1582-6. doi: 10.1038/leu.2015.343. Epub 2015 Dec 16. Leukemia. 2016. PMID: 26669973 Free PMC article. No abstract available. - Myeloid malignancies and the microenvironment.
Korn C, Méndez-Ferrer S. Korn C, et al. Blood. 2017 Feb 16;129(7):811-822. doi: 10.1182/blood-2016-09-670224. Epub 2016 Nov 15. Blood. 2017. PMID: 28064238 Free PMC article. - Targeting the bone marrow microenvironment in acute leukemia.
Karantanou C, Godavarthy PS, Krause DS. Karantanou C, et al. Leuk Lymphoma. 2018 Nov;59(11):2535-2545. doi: 10.1080/10428194.2018.1434886. Epub 2018 Feb 12. Leuk Lymphoma. 2018. PMID: 29431560 Review. - Targeting membrane trafficking in infection prophylaxis: dynamin inhibitors.
Harper CB, Popoff MR, McCluskey A, Robinson PJ, Meunier FA. Harper CB, et al. Trends Cell Biol. 2013 Feb;23(2):90-101. doi: 10.1016/j.tcb.2012.10.007. Epub 2012 Nov 17. Trends Cell Biol. 2013. PMID: 23164733 Review.
Cited by
- Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review. - The sulfonadyns: a class of aryl sulfonamides inhibiting dynamin I GTPase and clathrin mediated endocytosis are anti-seizure in animal models.
Odell LR, Jones NC, Chau N, Robertson MJ, Ambrus JI, Deane FM, Young KA, Whiting A, Xue J, Prichard K, Daniel JA, Gorgani NN, O'Brien TJ, Robinson PJ, McCluskey A. Odell LR, et al. RSC Med Chem. 2023 Apr 26;14(8):1492-1511. doi: 10.1039/d2md00371f. eCollection 2023 Aug 16. RSC Med Chem. 2023. PMID: 37593570 Free PMC article. - Discovery and Anticancer Activity of the Plagiochilins from the Liverwort Genus Plagiochila.
Bailly C. Bailly C. Life (Basel). 2023 Mar 10;13(3):758. doi: 10.3390/life13030758. Life (Basel). 2023. PMID: 36983914 Free PMC article. Review. - Prodrugs of the Archetypal Dynamin Inhibitor Bis-T-22.
Odell LR, Robertson MJ, Young KA, McGeachie AB, Quan A, Robinson PJ, McCluskey A. Odell LR, et al. ChemMedChem. 2022 Dec 16;17(24):e202200400. doi: 10.1002/cmdc.202200400. Epub 2022 Nov 9. ChemMedChem. 2022. PMID: 36351775 Free PMC article. - Overexpression of wild-type IL-7Rα promotes T-cell acute lymphoblastic leukemia/lymphoma.
Silva A, Almeida ARM, Cachucho A, Neto JL, Demeyer S, de Matos M, Hogan T, Li Y, Meijerink J, Cools J, Grosso AR, Seddon B, Barata JT. Silva A, et al. Blood. 2021 Sep 23;138(12):1040-1052. doi: 10.1182/blood.2019000553. Blood. 2021. PMID: 33970999 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases