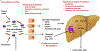Current Concepts, Opportunities, and Challenges of Gut Microbiome-Based Personalized Medicine in Nonalcoholic Fatty Liver Disease - PubMed (original) (raw)
Review
Current Concepts, Opportunities, and Challenges of Gut Microbiome-Based Personalized Medicine in Nonalcoholic Fatty Liver Disease
S R Sharpton et al. Cell Metab. 2021.
Abstract
Nonalcoholic fatty liver disease (NALFD) is now a leading cause of chronic liver disease worldwide, in part, as a consequence of rapidly rising levels of obesity and metabolic syndrome and is a major risk factor for cirrhosis, hepatocellular carcinoma, and liver-related mortality. From NAFLD stems a myriad of clinical challenges related to both diagnosis and management. A growing body of evidence suggests an intricate linkage between the gut microbiome and the pathogenesis of NAFLD. We highlight how our current knowledge of the gut-liver axis in NAFLD may be leveraged to develop gut microbiome-based personalized approaches for disease management, including its use as a non-invasive biomarker for diagnosis and staging, as a target for therapeutic modulation, and as a marker of drug response. We will also discuss current limitations of these microbiome-based approaches. Ultimately, a better understanding of microbiota-host interactions in NAFLD will inform the development of novel preventative strategies and precise therapeutic targets.
Keywords: biomarker; cirrhosis; fibrosis; microbiota; nonalcoholic steatohepatitis.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests S.S. declares no competing interests. B.S. has served as a consultant for Ferring Research Institute, Intercept Pharmaceuticals, HOST Therabiomics, and Patara Pharmaceuticals. B.S.’s institution University of California San Diego has received grant support from BiomX, NGM Biopharmaceuticals, CymaBay Therapeutics, Synlogic Operating Company, and Axial Biotherapeutics. R.K. serves as a consultant or advisory board member for Biota Technology, CoreBiome, GenCirq, Micronoma, DayTwo, Cybele Microbiome, CommenSe, and BiomeSense. R.L. serves as a consultant or advisory board member for Arrowhead Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, Galmed, Gemphire, Gilead, Glympse Bio, GNI, GRI Bio, Intercept, Ionis, Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Grail, Janssen, Madrigal Pharmaceuticals, NGM Biopharmaceuticals, Pfizer, pH Pharma, Prometheus, and Siemens. R.L. is also co-founder of Liponexus, Inc.
Figures
Figure 1.. Potential microbiome-based therapeutic interventions in nonalcoholic fatty liver disease (NAFLD).
Intestinal barrier dysfunction, in part related to leaky tight junctions, allows microbial products from gut bacteria, such as lipopolysaccharide, to enter the portal circulation and stimulate toll-like receptors (TLRs), leading to activation of the innate immune system. NAFLD is associated with perturbations in gut microbiota composition, often referred to as intestinal dysbiosis. Modulation of the gut microbiota is feasible via a diverse array of strategies including antimicrobials, prebiotics, probiotics, synbiotics, fecal microbiota transplantation (FMT), and bacteriophage therapy. Gut microbiota contribute to disease pathogenesis through metabolism of dietary substrates, xenobiotics, as well as host molecules (such as primary bile acids), resulting in the production of bioactive small molecules that enter the portal circulation and modulate microbiota-host interactions. Interventions specifically targeted to microbial metabolism may include postbiotics and small molecule inhibition and genetically engineered microbes to precisely alter the production of bacterial metabolites. Metabolites of particular interest in NAFLD pathogenesis include short-chain fatty acids (SCFAs), branched-chain amino acids (BCAAs), indoles, and secondary bile acids (derived from microbial metabolism of primary bile acids). Personalized dietary approaches may modulate dietary-microbiome crosstalk, with ensuing effects on both microbiota composition and metabolic output.
References
- Adams LA, Anstee QM, Tilg H, and Targher G. (2017). Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. - PubMed
- Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, Marchesi JR, McDonald JAK, Pechlivanis A, Barker GF, et al. (2020). Effects of Fecal Microbiota Transplantation With Oral Capsules in Obese Patients. Clin Gastroenterol Hepatol. - PubMed
Publication types
MeSH terms
Grants and funding
- UL1 TR001442/TR/NCATS NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- P30 DK120515/DK/NIDDK NIH HHS/United States
- R01 AA024726/AA/NIAAA NIH HHS/United States
- R37 AA020703/AA/NIAAA NIH HHS/United States
- U01 AA026939/AA/NIAAA NIH HHS/United States
- R01 DK106419/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical